Figure 2.
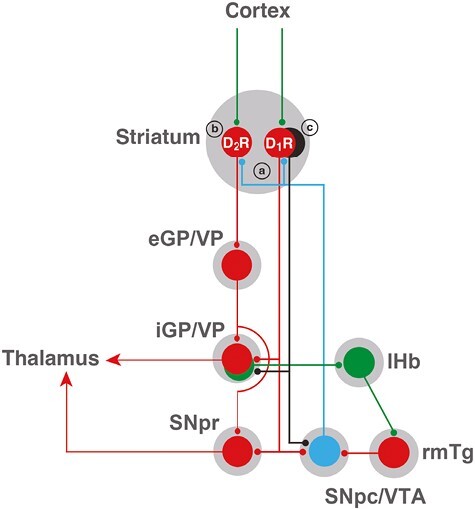
Scheme of cortical-striatal-pallidal-thalamic and cortical-striatal-pallidal-habenular basal ganglia circuits. Glutamatergic, GABAergic, and dopaminergic neurons are represented in green, red, and blue, respectively. D1R and D2R represent two main subtypes of striatal GABAergic neurons, the striatonigral and the striatopallidal neurons, expressing dopamine D1 receptors (D1Rs) and dopamine D2 receptors (D2Rs), respectively. The GABAergic striosomal D1R-MOR neurons are represented in black. It is postulated that an imbalance in the normal dopaminergic receptor activation of the D1R and D2R neurons and the specific increased activation of the striosomal D1R-MOR neurons, leading to inactivation of the pallidal-habenular neurons, mediates the akathisia/restlessness in RLS, NIA, and opioid withdrawal (see text). In NIA, this is dependent on an increased dopamine release (a), secondary to autoreceptor blockade, and a concomitant blockade of postsynaptic D2Rs (b). In RLS, there is also an increased dopamine release (a), secondary to glutamate release, a downregulation of D2Rs (b), and a concomitant increase in D1R sensitivity (c). With opioid withdrawal, there is no presynaptic component, but a significant increase in the sensitivity of the striosomal D1R-MOR neurons (c). Abbreviations: eGP/VP, external segment of the globus pallidus/ventral pallidum; iGP, internal segment of the globus pallidus; lHb, lateral habenula; rmTg, rostromedial tegmental nucleus; SNpc, substantia nigra pars compacta; SNpr, substantia nigra pars reticulata; VTA, ventral tegmental area. For simplification, the indirect connection of the GPe to the GPi and SNpr through the subthalamic nucleus is not included. Also, only the cortex is shown as an input to the striatum, but it also receives inputs from the midline and intralaminar thalamic nuclei, and the ventral striatum also receives inputs from the hippocampus and amygdala.
