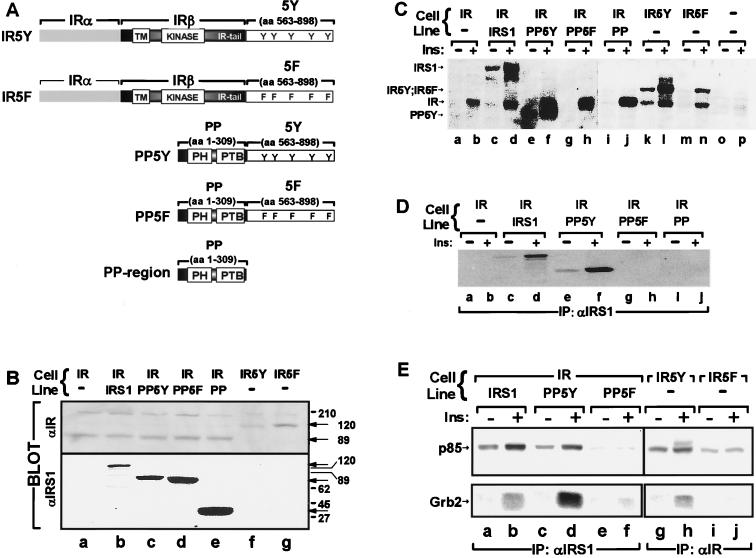
FIG. 1.
(A) Schematic representation of the chimeric molecules used in this study showing the various domains and phosphorylation sites. TM, transmembrane domain; 5Y region, amino acids (aa) 555 to 898 of wild-type IRS-1; 5F region, amino acids 555 to 898 of IRS-1 containing five Tyr→Phe substitutions. (B) Cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies against the COOH terminus of the IR (αIR) or the NH2 terminus of IRS-1 (αIRS1) as described in Materials and Methods. (C, D, and E) The indicated cell lines were starved for 4 h, stimulated with 100 nM insulin (Ins) for 5 min, and lysed as described in Materials and Methods. (C) Cell lysates directly resolved by SDS-PAGE and immunoblotted with αPY; (D) cell lysates immunoprecipitated (IP) with αIRS1, separated by SDS-PAGE, and immunoblotted with αPY; (E) cell lysates immunoprecipitated (IP) with αIRS1 or αIR, separated by SDS-PAGE, and immunoblotted with antiserum against p85 (top panel) or Grb-2 (bottom panel) as indicated. All results presented are representative of at least two separate experiments.