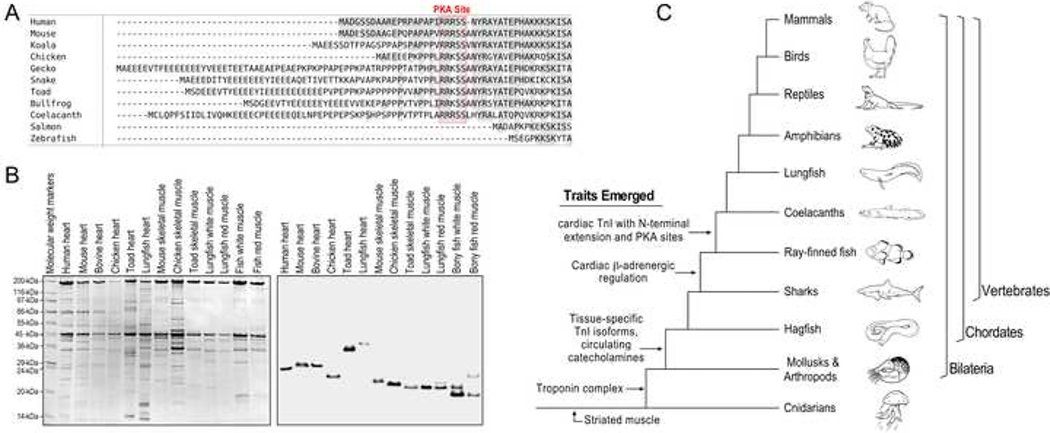
Fig. 4. Evolution of the N-terminal extension of cardiac TnI.
A, Amino acid sequence alignment of cardiac TnI of representative vertebrate species demonstrates that the N-terminal extension and the PKA phosphorylation sites are absent in ray-finned bony fishes but present in lobe-finned fishes such as the coelacanth as well as in amphibians, reptiles, birds, marsupials and mammals. B, Coomassie Brilliant Blue-stained SDS-gel (left) and anti-TnI mAb Western blot (right) of total protein extracts from cardiac and skeletal muscles of representative species show that lungfish (South American lungfish, Lepidosiren paradoxa) cardiac TnI has higher molecular weight than that of skeletal muscle TnIs and cardiac TnI of human, mouse, bovine, chicken and toad. Cardiac TnIs of those higher vertebrate species are known to have the N-terminal extension, suggesting lungfish cardiac TnI too contains an N-terminal extension. C, The tree of vertebrate evolution illustrates that striated muscle evolved in cnidarians, and troponin emerged in Bilaterian evolution. The prototype vertebrate hagfish possesses circulating catecholamines, and full β-adrenergic innervation of tissues is seen in some bony fishes where muscle type-specific isoforms of TnI also began to emerge. The cardiac TnI N-terminal extension and S23/S24 appears to have co-evolved in coelacanths and are present in all higher order vertebrates.