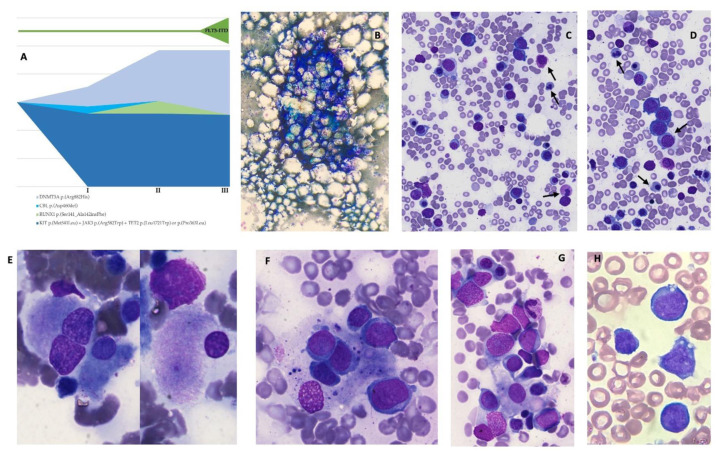
Figure 1. Bone marrow findings during different phases of the myeloid neoplasia.
A: Next Generation Sequencing (NGS) performed on bone marrow samples immediately before CAR-T treatment, at the onset of t-AML with myelodysplasia-related changes, and during the hyperleukocytosis phase with FLT3-ITD mutation. NGS reveals a pre-exhisting condition of CHIP with CBL p.(Asp460del), KIT p.(Met541Leu), DNMT3A p.(Arg882His), JAK3 p.(Arg582Trp), TET2 p.(Leu1721Trp) and TET2 p.(Pro363Leu) being expressed at various VAF. At the onset of t-AML, it is possible to observe the appearance of a RUNX1-mutated clone, which does not persist subsequently, and an expansion of the DNMT3A compound, together with the acquisition of FLT3-ITD mutation. B–C–D: 3 months after CAR-T. Bone marrow examination at the onset of severe trilinear cytopenia. The cellularity appears reduced with age. Granulocyte precursors are extremely rare and with dysmorphic features, resulting in relative erythroid hyperplasia. Erythoid precursors are represented in all maturative phases and show signs of diserytrhorpoiesis, with hemoglobinization defects, occasional binucleation, internuclear bridges, and asynchronous maturation. One macrophage makes contact with erythroid precursors. E–F–G: 6 months after CAR-T. Acute myeloid leukemia with myelodysplastic features and activated macrophages. Dysplastic megakaryocytes can be observed together with a considerable amount of heterogeneous blasts; blasts present with middle-to-large size, open chromatin, up to four nucleoli, and granulated basophilic cytoplasm. A huge number of activated macrophages with images of phagocytosis and cellular detritus can be observed, as well. H: 15 months after CAR-T. Acute myeloid leukemia with hyperleukocytosis and FLT3 mutation. WBC were 160x10^9/L, with large-size blasts with fine chromatin, one or more nucleoli, and basophil cytoplasm with occasional vacuoles.