Abstract
Background:
Adult survivors of childhood cancer are at elevated risk of morbidity and mortality compared to the general population, but their adherence to lifelong periodic surveillance is suboptimal. We aimed to examine adherence to surveillance guidelines for high-yield tests and identify risk factors for nonadherence in adult survivors of childhood cancer.
Methods:
In this retrospective, population-based cohort study, we used health care administrative data from Ontario, Canada, to identify adult survivors of childhood cancer diagnosed between 1986 and 2014 who were at elevated risk of therapy-related colorectal cancer, breast cancer, or cardiomyopathy. Using a Poisson regression framework, we assessed longitudinal adherence and predictors of adherence to the Children’s Oncology Group surveillance guideline.
Results:
Among 3241 survivors, 327 (10%), 234 (7%), and 3205 (99%) were at elevated risk for colorectal cancer, breast cancer, and cardiomyopathy, respectively. Within these cohorts, only 13%, 6%, and 53% were adherent to recommended surveillance as of February 2020. During a median follow-up of 7.8 years, the proportion of time spent adherent was 14% among survivors at elevated risk for colorectal cancer, 10% for breast cancer, and 43% for cardiomyopathy. Significant predictors of adherence varied across the risk groups, but higher comorbidity was associated with adherence to recommended surveillance.
Interpretation:
Survivors of childhood cancer in Ontario are rarely up to date for recommended surveillance tests. Tailored interventions beyond specialized clinics are needed to improve surveillance adherence.
Adult survivors of childhood cancer are at elevated risk of late morbidity and premature mortality (“late effects”), resulting from their treatment.1 As many as 80% of childhood cancer survivors (CCS) will develop a serious or life-threatening late effect by age 45 years.2 Of these late effects, cardiomyopathy1 and subsequent malignant neoplasms (including breast and colorectal cancers3–5) are among the leading causes of premature mortality. For example, female CCS who received chest radiation have a breast cancer risk comparable with that of females who carry a BRCA mutation.6 The risk of colorectal cancer in CCS is 2- to 3-fold higher than in the general population7 and as many as 50% of CCS who received anthracycline chemotherapy, radiation involving the heart, or both will develop clinical or subclinical cardiotoxicity.1
As risk-adapted surveillance can potentially reduce mortality,8,9 the Children’s Oncology Group offers long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers.10 Studies have shown the effectiveness and cost-effectiveness of adherence to these surveillance guidelines, hence their broad adoption by North American clinicians who care for CCS.9,11,12 However, guideline adherence among adult CCS and their health care providers is suboptimal,8,13–17 placing many CCS at substantial risk for preventable harm.
Our objective was to determine longitudinal surveillance adherence among adult CCS in Ontario, Canada, and to identify survivor and care provider characteristics associated with non-adherence, to inform future targeted interventions.
Methods
Setting
When CCS transition to adult care, they are given a summary of their treatment and the required surveillance testing. All adult CCS in Ontario are eligible to access long-term follow-up clinics (referred by the treating pediatric oncologist, or CCS can self-refer) located across 5 tertiary or quaternary provincial cancer centres (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231358/tab-related-content), but attendance is poor and has been declining.18,19 Many CCS drop out as they age.20,21 Some survivors have lifelong annual long-term follow-up clinic visits and others transition to their primary care physician. In the analysis, we included CCS who received a diagnosis between July 1, 1986, and Dec. 31, 2014.
Design
We conducted a retrospective, population-based cohort study of adult CCS in Ontario, Canada. The Reporting of Studies Conducted Using Observational Routinely-collected Health Data (RECORD) checklist is available in Appendix 2 (at www.cmaj.ca/lookup/doi/10.1503/cmaj.231358/tab-related-content).
Data sources
The Pediatric Oncology Group of Ontario Networked Information System (POGONIS)22 is a registry of all children and adolescents who received a cancer diagnosis before age 18 years and were treated at any of Ontario’s 5 pediatric cancer centres since 1986 (Appendix 1). Previous work has shown that POGONIS identifies more than 96% of Ontario children with cancer, younger than 14 years.22
We excluded CCS with a less than 5-year event-free period between their last childhood cancer event (latest of primary diagnosis, relapse, or subsequent malignant neoplasm before age 18 yr) and end of study (Feb. 28, 2020). Eligible CCS at elevated risk of cardiomyopathy (anthracycline, radiation to the heart, or both) were aged 18 years or older before the end of study. Eligible CCS at elevated risk of breast cancer (female survivor with radiation to the chest, axilla, or total body irradiation), colorectal cancer (radiation to the abdomen, chest, pelvis, spine, or total body irradiation), or both were age 25 years or older before the end of the study.
We excluded CCS if they had an invalid unique encoded identifier or missing data on sex, a follow-up time of 1 year or less, or emigrated out of Ontario.
We linked patients in POGONIS to population-based administrative databases (Appendix 3, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231358/tab-related-content) using unique encoded identifiers. Linked data sets were analyzed at ICES, an independent, nonprofit organization that analyzes data collected from administering Ontario’s publicly funded health care system. These databases have been validated23 and used extensively for health services research in Ontario (https://www.ices.on.ca/publications/). Databases capture hospital stays with diagnostic and procedure codes (Discharge Abstract Database), physician claims through the Ontario Health Insurance Plan (OHIP), outpatient ambulatory care visits (National Ambulatory Care Reporting System), cancer cases in Ontario (the Ontario Cancer Registry), basic demographics (Registered Persons Database), and use of breast cancer screening services (Ontario Breast Screening Program).
From POGONIS, we retrieved information on primary cancer diagnosis, diagnosis date, treatment (hematopoietic stem cell transplant, radiation, chemotherapy including anthracyclines), and relapse or subsequent malignant neoplasm before age 18 years. For CCS at elevated risk of colorectal or breast cancer, we categorized radiation as none, abdomen or pelvis only, breast and abdomen or pelvis, or breast only. For CCS at elevated risk of cardiomyopathy, we categorized treatment as anthracycline only, radiation involving the heart only, or radiation involving the heart and anthracycline.
We captured socioeconomic status at index through a composite measure of rurality and neighbourhood income. The Statistics Canada measure included 5 Census-derived neighbourhood income quintiles,24 with quintile 1 representing the lowest income level. Rural residence was considered a sixth category. We adhered to Statistics Canada’s recommendation not to report income quintiles in rural areas, owing to the variation of income within a single rural postal code, an approach that has been adopted by past studies.25–28
Using the OHIP database, we identified all primary care physician and long-term follow-up clinic visits in the year before the start date of each surveillance lookback period. We categorized Johns Hopkins’ Aggregated Diagnosis Groups (ADG) scores, representing measures of morbidity,29,30 as none, low (1–5), intermediate (6–9), and high (≥ 10).31
Outcomes
Five versions of the Children’s Oncology Group Long-Term Follow-Up Guidelines (Table 1 and Appendix 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231358/tab-related-content) have been produced. We assessed guideline adherence to surveillance for colorectal cancer, breast cancer, or cardiomyopathy because these outcomes involve 3 high-yield surveillance tests that are cost-effective in CCS.12,32
Table 1:
Children’s Oncology Group Long-Term Follow-Up Guidelines, version 5.010
| Breast cancer | Colorectal cancer | Cardiomyopathy |
|---|---|---|
|
Therapeutic exposure Any level of radiation exposure to the chest, axilla, or TBI Surveillance* Mammography — Yearly, beginning at age 25 yr or 8 yr post-radiation, whichever occurs last Breast MRI — Yearly, as an adjunct to mammography, beginning at age 25 yr or 8 yr post-radiation, whichever occurs last |
Therapeutic exposure Any level of radiation exposure to the abdomen, pelvis, spine (lumbar, sacral, whole), TBI Surveillance* Multitarget stool DNA test — performed every 3 yr, beginning at age 30 yr or 5 yr after radiation, whichever occurs last. Positive result should be followed up with a timely colonoscopy Colonoscopy — Performed every 5 yr, beginning at age 30 yr or 5 yr after radiation, whichever occurs last. |
Therapeutic exposure Anthracycline chemotherapy or radiation to a field that involves the heart Surveillance* Echocardiogram (or comparable imaging to evaluate cardiac function) starting at the completion of cancer therapy†
|
Note: MRI = magnetic resonance imaging, TBI = total body irradiation.
Recommendations as per version 5.0 — October 2018.10
Although echocardiographic screening is recommended to start during childhood, for the present analysis we have focused on screening that occurs once survivors become adults at age 18 years.
Current adherence analysis
Current adherence proportions for each surveillance category were calculated as the number of patients adherent on the last day of follow-up (using version 5 [V5] of the guidelines) divided by the total number of patients under follow-up at that time. To assess adherence at the beginning of follow-up, we included a lookback period (pre-index date) based on the surveillance test frequency. We also calculated adherence proportions in 2005, 2010, 2015, and 2019 and examined whether they consistently increased or decreased over time, using the Cochran–Armitage trend test. Proportions adherent for breast and colorectal cancer were compared between survivors newly eligible for the V5 guidelines (no radiation dose criteria for eligibility) and those who had been eligible in versions 1 to 4 (dose criteria for eligibility). Appendix 5 (available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231358/tab-related-content) provides observation window calculations.
Longitudinal analysis
We calculated follow-up time from each survivor’s index date, defined as the latter of age 18 years or 5 years after their last childhood cancer event (Appendix 5). We calculated longitudinal adherence based on when each guideline version was applicable. We measured adherence from the index date until an event in adulthood (relapse, subsequent malignant neoplasm), death, emigration out of Ontario, or the end of study.
We organized data as person–period–level, using a Poisson framework. The period reflected the number of surveillance windows during a patient’s follow-up. The first period began at the index date. Surveillance windows depended on the frequency of each recommended test (e.g., 1-year windows for a yearly screening test). We created fixed 1-year windows and determined the proportion adherent within each window using our algorithm. Patients could be censored midway through the last year; we used an offset term to account for this. We considered survivors to be adherent for 365 days after completing a test and then nonadherent until next test completion. Within each period, we calculated the proportion of time a patient was adherent as total days adherent divided by total days of follow-up in that period.
Predictors of adherence
Using the Poisson framework, we analyzed predictors of adherence with a Poisson multivariable regression model incorporating generalized estimating equations to account for multiple period data for each patient. The generalized estimating equations model allowed for possible overdispersion.
For each person in every period, we defined the outcome as total days adherent during the period. The regression model offset was the natural log of the patient’s follow-up time within that period. We entered variables significant (p < 0.10) in the univariate model for each at-risk group into a multivariable model. We added age at diagnosis, sex, and socioeconomic status a priori into the multivariable model regardless of significance; the latter 2 variables affect cancer screening in the general population.33,34 We included all covariates as baseline measures except for primary care physician and long-term follow-up clinic visits, which were updated over time.
Ethics approval
This study was exempted from ethics review and informed consent under Ontario’s Personal Health Information Protection Act.
Results
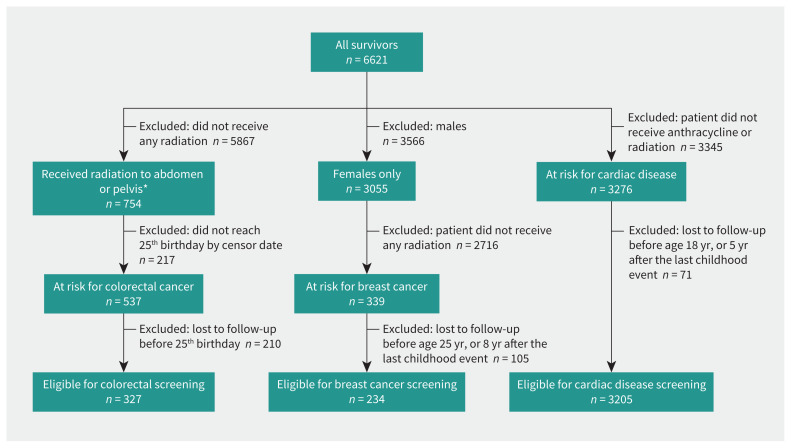
We identified 3241 adult survivors at elevated risk per the V5 guidelines (Figure 1). The number of survivors at elevated risk for 1, 2, or 3 late effects was 2806 (87%), 345 (11%), and 90 (3%), respectively. The number of survivors at risk of colorectal cancer, breast cancer, or cardiomyopathy was 327 (10%), 234 (7%), and 3205 (99%), respectively.
Figure 1:
Flow chart for childhood cancer survivors at risk for colorectal cancer, breast cancer, and cardiac complications. Note: A survivor can be in more than 1 group, at risk for cardiac and colorectal cancer. *In earlier versions of the Children’s Oncology Group Long-Term Follow-Up guidelines, only patients who received more than the specified amount of radiation were considered at risk for colorectal cancer. In the most recent version, there is no lower radiation dose limit.
Table 2 describes CCS baseline characteristics by risk type. Median follow-up time was 7.8 years (range 1.41–28.5 yr) and there were 31 476 person-years of follow-up. Median time from diagnosis date until the end of follow-up was 21.0 years (range 5.0–33.6 yr) and there were 79 149 person-years of follow-up. Reasons for censoring were death (n = 32), an adulthood cancer event (n = 149), or did not meet screening criteria before Feb. 28, 2020 (n = 205).
Table 2:
Distributions of baseline characteristics of high-risk survivors of childhood cancers by risk type, n = 3241
| Characteristic | No. (%)* of survivors at risk of colorectal cancer n = 327 |
No. (%)* of survivors at risk of breast cancer n = 234 |
No. (%)* of survivors at risk of cardiomyopathy n = 3205 |
|---|---|---|---|
| Age at first diagnosis, yr | |||
| Median (IQR) | 11 (6–14) | 13 (8–15) | 9 (4–13) |
| 0–4 | 53 (16.2) | 28 (12.0) | 930 (29.0) |
| 5–11 | 115 (35.2) | 60 (25.6) | 1094 (34.1) |
| 12–18 | 159 (48.6) | 146 (62.4) | 1181 (36.8) |
| Sex | |||
| Female | 130 (39.8) | 234 (100.0) | 1377 (43.0) |
| Male | 197 (60.2) | 0 (0) | 1828 (57) |
| Rurality and neighbourhood income | |||
| Rural | 33 (10.2) | 23 (10.0) | 366 (11.5) |
| Urban and income quintile 1 | 47 (14.6) | 33 (14.3) | 406 (12.8) |
| Urban and income quintile 2 | 51 (15.8) | 41 (17.8) | 529 (16.7) |
| Urban and income quintile 3 | 69 (21.4) | 43 (18.7) | 597 (18.8) |
| Urban and income quintile 4 | 50 (15.5) | 34 (14.8) | 593 (18.7) |
| Urban and income quintile 5 | 72 (22.4) | 55 (23.9) | 669 (21.1) |
| Missing | 0 (0) | 5 (0.4) | 12 (0.4) |
| Diagnosis | |||
| Leukemias | 40 (12.2) | 25 (10.7) | 1402 (43.7) |
| Lymphomas | 137 (41.9) | 149 (63.7) | 939 (29.3) |
| Central nervous system tumours | 65 (19.9) | 36 (15.4) | 138 (4.3) |
| Solid tumours | 80 (24.8) | 15 (6.7) | 749 (23.2) |
| Period of diagnosis | |||
| 1986–1996 | 243 (74.3) | 117 (50.0) | 1309 (40.8) |
| 1997–2007 | 84 (25.7) | 98 (41.9) | 1418 (44.2) |
| 2008–2014 | 0 (0) | 19 (8.1) | 478 (14.9) |
| Anthracycline dose† | |||
| None | 99 (30.3) | 59 (25.2) | 216 (6.7) |
| < 250 mg/m2 | 152 (46.5) | 136 (58.1) | 2148 (67.0) |
| ≥ 250 mg/m2 | 76 (23.2) | 39 (16.7) | 841 (26.2) |
| Transplant | |||
| Allogenic | 25 (7.6) | 18 (7.7) | 179 (5.6) |
| Autologous | 11 (3.4) | 10 (4.3) | 149 (4.6) |
| None | 291 (89.0) | 206 (88.0) | 2877 (89.8) |
| Radiation | |||
| Breast and abdomen or pelvis | 255 (78.0) | 151 (64.5) | 491 (15.3) |
| Breast only | 0 (0) | 83 (35.5) | 238 (7.4) |
| Abdomen or pelvis only | 72 (22.0) | 0 (0) | 171 (5.3) |
| No radiation to any body region | 0 (0) | 0 (0) | 2305 (71.9) |
| Follow-up time, yr | |||
| Median (IQR) | 1.41 (1.4–1.4) | 1.95 (1.41–6.7) | 9.33 (4.2–14.6) |
| Range | 0–13.5 | 0.1–11.4 | 0.1–17.0 |
| Total person-years contributed | 601.8 | 959.5 | 29 915.1 |
Note: IQR = interquartile range.
Unless otherwise specified. A survivor can be in more than 1 group.
Doxorubicin equivalents.
Current adherence
At the end of follow-up, 3241 survivors remained in the cohort, with 13%, 6%, and 53% adherent to colorectal cancer, breast cancer, and cardiomyopathy surveillance recommendations, respectively (Table 3). The Cochran–Armitage trend test indicated that the proportions differed over time for cardiomyopathy, breast, and colorectal adherence (p < 0.05, Table 4). Over time, adherence proportions increased for colorectal cancer and cardiomyopathy but decreased for breast cancer (Table 4). Breast cancer surveillance adherence proportions in 2019 were lower than in previous years. Adherence proportions for mammograms also decreased over time. At last follow-up, only 6% of elevated-risk females had completed both mammogram and magnetic resonance imaging (MRI) and were considered adherent; 10% had completed a mammogram but not an MRI. Breast and colorectal cancer surveillance adherence was not significantly different between those who became newly eligible for the V5 guidelines compared with those eligible in previous versions (Appendix 6, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231358/tab-related-content).
Table 3:
Proportion of high-risk childhood cancer survivors adherent on the last day of follow-up, by risk type (up to Feb. 28, 2020)*
| Characteristic | Survivors at risk of colorectal cancer (%) n = 327 |
Survivors at risk of breast cancer (%) n = 234 |
Survivors at risk of cardiomyopathy (%) n = 3205 |
|---|---|---|---|
| Overall | 12.8 | 6.4 | 52.7 |
| Age at first diagnosis, yr | |||
| 0–4 | 5.7 | 0 | 59.6 |
| 5–11 | 8.7 | 5.0 | 54.8 |
| 12–18 | 18.2 | 8.2 | 45.3 |
| Age at end of follow-up, yr | |||
| 18–24 | 0 | 7.5 | 72.5 |
| 25–29 | 5.4 | 6.0 | 54.0 |
| 30–34 | 17.4 | 4.3 | 42.0 |
| 35–39 | 21.3 | 9.7 | 37.8 |
| 40–44 | 21.1 | 0 | 29.9 |
| 45–49 | – | – | 16.9 |
| 50–54 | – | – | 0 |
| Sex | |||
| Female | 21.5 | 6.4 | 56.9 |
| Male | 7.1 | NA | 49.5 |
| Rurality and neighbourhood income, end of follow-up | |||
| Rural | 6.7 | 0 | 54.8 |
| Urban and income quintile 1 | 15.0 | 0 | 47.0 |
| Urban and income quintile 2 | 14.3 | 8.8 | 55.5 |
| Urban and income quintile 3 | 14.3 | 7.0 | 55.9 |
| Urban and income quintile 4 | 16.4 | 7.3 | 51.5 |
| Urban and income quintile 5 | 9.8 | 14.3 | 56.5 |
| Diagnosis | |||
| Leukemias | 10.0 | 4.0 | 57.9 |
| Lymphomas and neoplasms | 16.1 | 8.1 | 49.6 |
| Solid tumours and other categories | 10.7 | 3.3 | 47.5 |
| Period of diagnosis | |||
| 1986–1996 | 13.6 | 6.8 | 40.6 |
| 1997–2007 | 10.7 | 4.1 | 58.9 |
| 2008–2014 | – | 15.8 | 67.4 |
| Anthracycline dose | |||
| None | 8.1 | 5.1 | 38.4 |
| < 250 mg/m2 | 15.1 | 5.9 | 54.8 |
| ≥ 250 mg/m2 | 14.5 | 10.3 | 50.8 |
| Transplant | |||
| Allogenic | 12.0 | 5.6 | 49.2 |
| Autologous | 9.1 | 0 | 46.3 |
| None | 13.1 | 6.8 | 53.2 |
| Radiation | |||
| Breast and abdomen or pelvis | 13.3 | 6.6 | 39.9 |
| Breast only | 6.0 | 51.3 | |
| Abdomen or pelvis only | 11.1 | – | 57.3 |
| No radiation to any body region | – | – | 55.2 |
| ADG | |||
| None | 0 | 0 | 8.5 |
| Low, 1–5 | 9.5 | 4.2 | 54.5 |
| Intermediate, 6–9 | 15.3 | 8.0 | 62.0 |
| High, ≥ 10 | 28.8 | 9.2 | 67.9 |
| No. of primary care visits in the yr before end of follow-up | |||
| No visits | 1.9 | 3.5 | 35.2 |
| 1–3 | 17.2 | 3.7 | 59.0 |
| ≥ 4 | 18.5 | 10.6 | 60.2 |
| LTFU clinic visit in the yr before end of follow-up | |||
| No | 9.2 | 5.8 | 39.3 |
| Yes | 30.4 | 8.1 | 85.5 |
Note: – = no survivors in these categories, ADG = Aggregated Diagnosis Groups, LTFU = long-term follow-up.
A lookback period before the last day of follow-up assessed whether the survivor was adherent. We calculated rates by taking the number of patients who were adherent on the last day of follow-up divided by the total number of eligible survivors.
Table 4:
Proportion of high-risk childhood cancer survivors adherent by risk type, over time (on the last day of follow-up or end of the year)*
| Lookback from | N | Colorectal cancer | Breast cancer | Breast cancer (mammography only) | Cardiomyopathy | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| n eligible | Proportion adherent (95% CI) | n eligible | Proportion adherent (95% CI) | n eligible | Proportion adherent (95% CI) | n eligible | Proportion adherent (95% CI) | ||
| Dec. 31, 2005 | 1001 | – | 2.4 (0.1–13.6) | 58 | 19.0 (9.5–33.9) | 58 | 19.0 (9.5–33.9) | 980 | 30.8 (27.4–34.5) |
|
| |||||||||
| Dec. 31, 2010 | 1785 | 42 | 0 (0) | 90 | 7.8 (3.1–16.0) | 90 | 15.6 (8.5–26.1) | 1741 | 39.2 (36.3–42.2) |
|
| |||||||||
| Dec. 31, 2015 | 2544 | – | 11.9 (3.9–27.8) | 125 | 10.4 (5.5–17.8) | 125 | 16.0 (9.8–24.7) | 2490 | 47.3 (44.7–50.1) |
|
| |||||||||
| Dec. 31, 2019 | 3241 | 323 | 13.6 (9.9–18.3) | 231 | 5.6 (3.0–9.62) | 231 | 9.5 (6.0–14.4) | 3205 | 53.0 (50.5–55.5) |
|
| |||||||||
| Feb. 28, 2020 | 3241 | 327 | 12.8 (9.3–17.4) | 234 | 6.4 (3.6–10.6) | 234 | 9.8 (6.2–14.8) | 3205 | 52.7 (50.2–55.2) |
|
| |||||||||
| p value from Cochran– Armitage trend test | 0.003 | 0.003 | 0.01 | < 0.0001 | |||||
Note: CI = confidence interval.
A lookback period before the last day of follow-up assessed whether the survivor was adherent. We calculated proportions by taking the number of patients who were adherent on the last day of follow-up, divided by the total number of eligible survivors. We included data on patients before this date until they were taken out of the analysis, as described in the Methods.
Longitudinal adherence
The proportion of time adherent to surveillance guidelines was highest for survivors at elevated risk for cardiomyopathy (43%), followed by colorectal cancer (14%) and breast cancer (10%) (Table 5). Table 6, Table 7, and Table 8 describe factors associated with adherence. Greater adherence to colorectal cancer surveillance was associated with older age at diagnosis, female sex, and higher ADG scores. Greater adherence to breast cancer surveillance was associated with older age at diagnosis, more recent period of diagnosis, no transplant, and higher ADG scores. Greater adherence to cardiomyopathy surveillance was associated with younger age at diagnosis, female sex, highest income neighbourhood, more recent period of diagnosis, radiation and anthracyclines, higher anthracycline dose, no autologous transplant, higher ADG scores, more primary care physician visits, and a long-term follow-up clinic visit. Survivors who attended a long-term follow-up clinic in the previous year had generally poor adherence but better than the rest of the cohort: proportion of time adherent was 71% for those at elevated risk for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer, compared with 27%, 11%, and 6%, respectively.
Table 5:
Proportion of time that childhood cancer survivors spent adherent from Mar. 1, 2003, to Feb. 28, 2020, by risk type*
| Characteristic | Colorectal cancer (%) n = 327 |
Breast cancer (%) n = 234 |
Cardiomyopathy (%) n = 3205 |
|---|---|---|---|
| Overall | 13.9 | 10.0 | 42.5 |
| Age at first diagnosis, yr | |||
| 0–4 | 6.4 | 0 | 45.5 |
| 5–11 | 11.7 | 5.0 | 46.3 |
| 12–18 | 16.3 | 13.0 | 37.1 |
| Current age, end of follow-up, yr | |||
| 18–24 | 0 | 6.2 | 72.9 |
| 25–29 | 6.6 | 10.8 | 56.4 |
| 30–34 | 17.3 | 2.9 | 41.2 |
| 35–39 | 17.6 | 25.6 | 29.4 |
| 40–44 | 13.1 | 3.7 | 20.1 |
| 45–49 | – | – | 16.0 |
| 50–54 | – | – | 0 |
| Sex | |||
| Female | 20.9 | 10.1 | 46.4 |
| Male | 9.5 | NA | 39.6 |
| Rurality and neighbourhood income | |||
| Rural | 16.3 | 6.0 | 42.8 |
| Urban and income quintile 1 | 13.0 | 2.3 | 40.5 |
| Urban and income quintile 2 | 16.1 | 11.4 | 45.0 |
| Urban and income quintile 3 | 11.8 | 11.6 | 43.5 |
| Urban and income quintile 4 | 18.5 | 15.3 | 43.7 |
| Urban and income quintile 5 | 10.4 | 14.9 | 44.1 |
| Missing | 16.3 | 6.0 | 42.8 |
| Diagnosis | |||
| Leukemias | 11.5 | 0.4 | 48.0 |
| Lymphomas and neoplasms | 11.8 | 14.4 | 41.0 |
| Solid tumours (and other categories) | 15.6 | 1.9 | 34.2 |
| Period of diagnosis | |||
| 1986–1996 | 14.4 | 12.7 | 32.3 |
| 1997–2007 | 11.0 | 7.1 | 55.0 |
| 2008–2014 | – | 1.3 | 67.4 |
| Anthracycline dose | |||
| None | 10.1 | 3.5 | 42.0 |
| < 250 mg/m2 | 13.2 | 14.3 | 45.8 |
| ≥ 250 mg/m2 | 23.9 | 10.1 | 35.2 |
| Transplant | |||
| Allogenic | 13.7 | 0.5 | 38.9 |
| Autologous | 10.9 | 3.5 | 36.0 |
| None | 13.9 | 10.7 | 43.1 |
| Radiation | |||
| Breast and abdomen or pelvis | 12.6 | 11.1 | 30.9 |
| Breast only | – | 8.0 | 43.2 |
| Abdomen or pelvis only | 18.9 | – | 41.9 |
| No radiation to any body region | – | – | 44.5 |
| ADG | |||
| None | 0.7 | 1.3 | 16.8 |
| Low | 10.0 | 9.6 | 44.7 |
| Intermediate | 23.0 | 13.5 | 49.0 |
| High | 22.6 | 11.3 | 51.4 |
| Median no. of primary care visits (in the previous yr) for every follow-up yr | |||
| No visits | 3.3 | 1.5 | 25.8 |
| 1–3 | 16.9 | 10.3 | 47.6 |
| ≥ 4 | 20.8 | 14.2 | 47.0 |
| LTFU clinic visit (in the previous yr) for every follow-up yr | |||
| No | 10.5 | 6.5 | 27.2 |
| Yes | 26.7 | 15.0 | 71.5 |
Note: – = no survivors in this group, ADG = Aggregated Diagnosis Groups, LTFU = long-term follow-up, NA = not applicable.
Proportion of time adherent was calculated by taking the total amount of time that a patient was adherent throughout the follow-up period and dividing by the total days of follow-up starting from when a patient started screening eligibility. Then the value was multiplied by 100 to obtain the final rate in person-days.
Table 6:
Longitudinal adherence to colorectal cancer surveillance guidelines from Mar. 1, 2003, to Feb. 28, 2020, in327 survivors of childhood cancer — univariable and multivariable rate ratios and 95% confidence intervals from generalized estimating equations Poisson regression*
| Characteristic | Univariable RR (95% CI) | Multivariable RR (95% CI) |
|---|---|---|
| Age at diagnosis, yr | 1.05 (0.99–1.12) | 1.07 (1.01–1.14) |
| Sex | ||
| Female v. male | 2.75 (1.52–4.98) | 1.86 (1.05–3.31) |
| Rurality and neighbourhood income | ||
| Rural v. urban and income quintile 5 | 0.73 (0.24–2.24) | 1 (0.33–3.04) |
| Urban and income quintile 1 v. urban and income quintile 5 | 1 (0.48–2.08) | 1.25 (0.57–2.73) |
| Urban and income quintile 2 v. urban and income quintile 5 | 0.92 (0.47–1.78) | 0.93 (0.47–1.83) |
| Urban and income quintile 3 v. urban and income quintile 5 | 0.85 (0.41–1.77) | 0.94 (0.41–2.18) |
| Urban and income quintile 4 v. urban and income quintile 5 | 0.97 (0.47–2.01) | 1.07 (0.51–2.21) |
| Period of diagnosis | ||
| 1997–2007 v. 1986–1996 | 0.76 (0.38–1.52) | – |
| 2008–2014 v. 1986–1996 | – | – |
| Anthracycline dose | ||
| < 250 mg/m2 v. none | 1.39 (0.69–2.78) | 1.2 (0.6–2.4) |
| ≥ 250 mg/m2 v. none | 2.18 (1.04–4.58) | 1.75 (0.89–3.45) |
| Transplant | ||
| Allogenic v. none | 1.03 (0.35–3.06) | – |
| Autologous v. none | 0.79 (0.12–5.16) | – |
| Radiation | ||
| No radiation to any body region v. breast and abdomen or pelvis | No patients in this category | – |
| Abdomen or pelvis only v. breast and abdomen or pelvis | 1.21 (0.61–2.38) | – |
| Breast only v. breast and abdomen or pelvis | No patients in this category | – |
| ADG, categorical | ||
| High v. none | 10.72 (4.65–24.69) | 8.58 (2.87–25.59) |
| Intermediate v. none | 9.07 (4.47–18.39) | 7.51 (2.99–18.88) |
| Low v. none | 4.29 (1.9–9.66) | 3.8 (1.6–9.05) |
| PCP visits in the past yr | ||
| 1–3 PCP visits v. no PCP visits | 2.54 (1.42–4.53) | 1.3 (0.59–2.85) |
| ≥ 4 PCP visits v. no PCP visits | 2.3 (1.09–4.86) | 0.93 (0.33–2.63) |
| LTFU clinic visit in the past yr | ||
| Yes v. no | 2.01 (1.29–3.11) | 1.33 (0.89–1.98) |
Note: ADG = Aggregated Diagnosis Groups, CI = confidence interval, LTFU = long-term follow-up, PCP = primary care physician, RR = rate ratio.
Age, sex, rurality, anthracycline dose, ADG, PCP visits in the past year.
Table 7:
Longitudinal adherence to breast cancer surveillance guidelines from Mar. 1, 2003, to Feb. 28, 2020, for 234 survivors — univariable and multivariable rate ratios and 95% confidence intervals from Poisson regression*
| Characteristic | Univariable RR (95% CI) | Multivariable RR (95% CI) |
|---|---|---|
| Age at diagnosis, yr | 1.15 (1.05–1.25) | 1.09 (1.01–1.18) |
| Rurality and neighbourhood income | ||
| Rural v. urban and income quintile 5 | 1.02 (0.38–2.74) | 0.85 (0.32–2.25) |
| Urban and income quintile 1 v. urban and income quintile 5 | 1.2 (0.51–2.81) | 1.09 (0.54–2.23) |
| Urban and income quintile 2 v. urban and income quintile 5 | 1.01 (0.36–2.87) | 0.95 (0.45–2.03) |
| Urban and income quintile 3 v. urban and income quintile 5 | 0.46 (0.14–1.54) | 0.51 (0.23–1.13) |
| Urban and income quintile 4 v. urban and income quintile 5 | 0.65 (0.26–1.61) | 0.59 (0.31–1.12) |
| Period of diagnosis | ||
| 1997–2007 v. 1986–1996 | 0.71 (0.33–1.52) | 0.44 (0.23–0.85) |
| 2008–2014 v. 1986–1996 | 0.14 (0.02–0.84) | 0.09 (0.02–0.39) |
| Anthracycline dose | ||
| < 250 mg/m2 v. none | 3.36 (0.96–11.73) | 2.43 (0.72–8.17) |
| ≥ 250 mg/m2 v. none | 2.8 (0.74–10.63) | 2.15 (0.63–7.31) |
| Transplant | ||
| Allogenic v. none | 0.08 (0.01–0.6) | 0.14 (0.02–1.18) |
| Autologous v. none | 0.3 (0.08–1.15) | 0.13 (0.04–0.41) |
| Radiation | ||
| No radiation to any body region v. breast and abdomen or pelvis | No patients in this category | – |
| Abdomen or pelvis only vs breast and abdomen or pelvis | No patients in this category | – |
| Breast only v. breast and abdomen or pelvis | 0.65 (0.31–1.38) | – |
| ADG, categorical | ||
| High v. none | 5.14 (1.5–17.67) | 3.21 (1.25–8.25) |
| Intermediate v. none | 5.91 (2.21–15.79) | 3.82 (1.9–7.7) |
| Low v. none | 4.52 (1.82–11.25) | 2.63 (1.32–5.21) |
| PCP visits in the past yr | ||
| 1–3 PCP visits v. no PCP visits | 1.35 (0.9–2.04) | 1.15 (0.75–1.74) |
| ≥ 4 PCP visits v. no PCP visits | 1.11 (0.63–1.96) | 0.95 (0.55–1.65) |
| LTFU clinic visit in the past yr | ||
| Yes v. no | 1.56 (0.66–3.68) | 1.59 (0.88–2.89) |
Note: ADG = Aggregated Diagnosis Groups, CI = confidence interval, LTFU = long-term follow-up, PCP = primary care physician, RR = rate ratio.
Age, rurality, period of diagnosis, anthracycline dose, transplant, ADG, PCP visits in the past year, LTFU clinic visit.
Table 8:
Longitudinal adherence — univariable and multivariable rate ratios and 95% confidence intervals from Poisson regression for adherence to cardiomyopathy surveillance guidelines from Mar. 1, 2003, to Feb. 28, 2020, for 3205 survivors*
| Characteristic | Univariable RR (95% CI) | Multivariable RR (95% CI) |
|---|---|---|
| Age at diagnosis, yr | 0.99 (0.98–0.99) | 0.97 (0.96–0.97) |
| Sex | ||
| Female v. male | 1.09 (1.03–1.15) | 1.03 (0.98–1.07) |
| Rurality and neighbourhood income | ||
| Rural v. urban and income quintile 5 | 1.03 (0.97–1.1) | 1.02 (0.96–1.09) |
| Urban and income quintile 1 v. urban and income quintile 5 | 0.91 (0.86–0.97) | 0.93 (0.87–0.99) |
| Urban and income quintile 2 v. urban and income quintile 5 | 0.96 (0.91–1.01) | 0.97 (0.91–1.02) |
| Urban and income quintile 3 v. urban and income quintile 5 | 0.98 (0.93–1.03) | 0.98 (0.93–1.03) |
| Urban and income quintile 4 v. urban and income quintile 5 | 0.97 (0.92–1.01) | 0.96 (0.92–1.01) |
| Period of diagnosis | ||
| 1997–2007 v. 1986–1996 | 1.79 (1.67–1.91) | 1.64 (1.55–1.73) |
| 2008–2014 v. 1986–1996 | 2.05 (1.9–2.2) | 2.16 (2.01–2.32) |
| Anthracycline dose | ||
| < 250 mg/m2 v. none | 1.52 (1.28–1.81) | 0.96 (0.81–1.14) |
| ≥ 250 mg/m2 v. none | 1.11 (0.93–1.34) | 0.77 (0.65–0.92) |
| Transplant | ||
| Allogenic v. none | 0.93 (0.82–1.06) | 0.99 (0.9–1.09) |
| Autologous v. none | 0.82 (0.7–0.95) | 0.9 (0.79–1.03) |
| Radiation and anthracycline | ||
| Anthracycline only v. radiation only | 1.86 (1.5–2.31) | 1.97 (1.51–2.55) |
| Radiation and anthracycline v. radiation only | 1.51 (1.21–1.88) | 1.64 (1.26–2.15) |
| ADG | ||
| High v. none | 2.84 (2.62–3.06) | 2.81 (2.53–3.12) |
| Intermediate v. none | 2.65 (2.46–2.85) | 2.63 (2.38–2.91) |
| Low v. none | 2.34 (2.18–2.51) | 2.38 (2.16–2.63) |
| PCP visits in the past yr | ||
| 1–3 PCP visits v. no PCP visits | 1.16 (1.13–1.19) | 1.04 (1.01–1.07) |
| ≥ 4 PCP visits v. no PCP visits | 1.22 (1.19–1.26) | 1.05 (1.02–1.08) |
| LTFU clinic visit in the past yr | ||
| Yes v. no | 1.4 (1.36–1.44) | 1.34 (1.3–1.37) |
Note: ADG = Aggregated Diagnosis Groups, CI = confidence interval, LTFU = long-term follow-up, PCP = primary care physician, RR = rate ratio.
Age, sex, rurality, period of diagnosis, anthracycline dose, transplant, radiation and anthracycline, ADG, PCP visits in the past year, LTFU visit.
Interpretation
In this population-based cohort of 3241 adult survivors of childhood cancer followed for up to 29 years with comprehensive outcome assessments using administrative data, survivors at elevated risk for cardiomyopathy, breast cancer, or colorectal cancer spent a majority of time nonadherent to surveillance guideline recommendations. Although adherence to cardiomyopathy and colorectal cancer surveillance increased over time, adherence to breast cancer surveillance decreased.
These findings mirror the low proportions of adherence in the United States.35 In Canada, health insurance is rarely a barrier to accessing surveillance, although indirect costs (e.g., time off work, travel) may affect test completion.36,37 Earlier studies found that a lack of knowledge about late effects risks and surveillance recommendations among survivors,38,39 family physicians,40,41 and specialists42 are substantial barriers to adherence. Our results support this: despite primary care physician visits, survivors’ adherence to breast and colorectal cancer surveillance guidance was low. Primary care physician visits were associated only with higher adherence to cardiomyopathy surveillance, although others have found an association with adherence to colorectal surveillance.16 This may reflect practical barriers; compared with other surveillance tests, echocardiography is more readily available and less restrictive for primary care physicians in Ontario to arrange than a multitarget stool DNA test.
Across the surveillance tests, higher comorbidity was the most robust (rate ratio [RR] > 2) and consistent predictor of better adherence. In the general population, some studies have found higher adherence to cancer screening guidelines among those with self-reported poor health43 and chronic illnesses,44,45 but others have reported mixed results.46 Perhaps survivors with greater morbidity and their medical teams are more motivated to conduct investigations. Rurality and income quintile did not significantly predict colorectal and breast cancer surveillance adherence, but with small numbers, there may be undetected differences. For cardiomyopathy surveillance, CCS in the lowest income quintile had lower adherence (RR = 0.93, p = 0.02).
Long-term follow-up clinic attendance was a significant predictor of CCS completing cardiomyopathy surveillance. These specialized clinics often have access to surveillance tests and are staffed by physicians who are unlikely to be more familiar with surveillance recommendations for CCS than the general population of physicians.18 Survivors who attend such clinics may also be more inclined to seek preventive care, driving higher surveillance rates. However, even survivors who attended a long-term follow-up clinic had generally poor adherence to all 3 surveillance guidelines. Long-term follow-up clinic physicians can recommend or order tests, but it is up to patients to complete them. Our study did not assess potential barriers to patients’ ability to access screening.
Older age at diagnosis was associated with greater adherence to breast and colorectal surveillance, but the inverse was observed for cardiomyopathy surveillance. Perhaps survivors who receive a diagnosis at an older age are more aware of their cancer and are more motivated to complete surveillance. Although female sex was a predictor of greater adherence to cardiomyopathy and colorectal cancer surveillance, the literature is inconsistent regarding sex as a predictor for screening adherence.35,46–48 A more recent period of diagnosis was associated with greater adherence to breast and cardiomyopathy surveillance, perhaps because physicians are more aware of the Children’s Oncology Group guidelines (first published in 2003).
The low surveillance rates we observed for colorectal cancer are consistent with findings from other at-risk populations.47,49,50 Fear of bowel preparation is a substantial barrier.50,51 Other surveillance approaches might be more acceptable to survivors.52 The V5 guidelines suggest that a fecal immunochemical test is a reasonable alternative to colonoscopy, but it is generally not acceptable to high-risk patients.
Our research suggests that further work on rural residence and lower socioeconomic status as predictors of surveillance adherence can elucidate barriers to screening. There is also a need to evaluate the trade-offs in costs, accessibility, and usability of surveillance tests against accuracy in adult CCS. Our findings demonstrate a need to support patients and clinicians to improve adherence to surveillance guidelines among CCS. This responsibility must be shared between the cancer care system, particularly the provincial pediatric cancer survivor network, as well as the patients themselves, through advocacy and other survivor support groups.
Limitations
We examined survivors in a province with survivorship clinics and publicly funded health insurance, which may affect generalizability to other jurisdictions. However, other jurisdictions may have even more barriers to obtaining recommended testing, so these poor adherence proportions may represent best-case scenarios. Importantly, we derived outcomes from administrative data instead of direct patient assessment. They do not capture CCS who emigrated out of the province or the purpose of a test (surveillance or diagnostic). Survivors of childhood cancer who turned 18 years old before 2018 were not discharged from pediatric care with knowledge of the updated guidelines, complicating their ability to adhere to the most recent guidelines. Also, other methods are possible for surveillance (e.g., ultrasound for breast cancer screening), but would not be guideline adherent.
Finally, young adults may avoid or disengage from the health care system owing to the trauma of their cancer experience;21,53,54 administrative databases cannot capture such factors.
Conclusion
Surveillance for late effects in adult survivors of childhood cancer is poor, placing many survivors at risk for preventable harm. To increase surveillance among this elevated-risk population, screening recommendations need to consider and address barriers to completing surveillance tests. Surveillance approaches that meet the needs of survivors and their physicians are important to help CCS stay healthy in adulthood.
Footnotes
Competing interests: Jennifer Shuldiner reports receiving a Canadian Institutes of Health Research (CIHR) Health System Impact Post-doctoral Fellowship, in support of the current manuscript. Noah Ivers is supported by a CIHR Tier 2 Canada Research Chair in Implementation of Evidence-based Practice (paid to institution). Paul Nathan is supported by a CIHR Foundation Grant and a grant from the United States Department of Defense (both paid to institution). No other competing interests were declared.
This article has been peer reviewed.
Contributors: Jennifer Shuldiner, Paul Nathan, and Noah Ivers contributed to the conception and design of the work. Rinku Sutradhar and Cindy Lau acquired the data. Paul Nathan, Noah Ivers, Rinku Sutradhar, Cindy Lau, Nida Shah, and Emily Lam contributed to the analysis and interpretation of data. Jennifer Shuldiner drafted the manuscript. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care. This study also received funding from the Canadian Institutes of Health Research. Parts of this material are based on data and information compiled and provided by the Ontario MOH. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. This research was facilitated by the Pediatric Oncology Group of Ontario’s Networked Information System, financially supported by Ontario’s Ministry of Health and Long-Term Care.
Data sharing: The data set from this study is held securely in coded form at ICES. While legal data-sharing agreements between ICES and data providers (e.g., health care organizations and government) prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca). The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
References
- 1.Lipshultz SE, Adams MJ, Colan SD, et al. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Basic Cardiovascular Sciences, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity and Metabolism. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 2013;128:1927–95. [DOI] [PubMed] [Google Scholar]
- 2.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309:2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia S, Yasui Y, Robison LL, et al. Late Effects Study Group. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol 2003;21:4386–94. [DOI] [PubMed] [Google Scholar]
- 4.Jenkinson HC, Hawkins M, Stiller C, et al. Long-term population-based risks of second malignant neoplasms after childhood cancer in Britain. Br J Cancer 2004;91:1905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: findings from the childhood cancer survivor study cohort. J Clin Oncol 2009;27:2356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol 2014;32:2217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teepen JC, Kok JL, van Leeuwen FE, et al. DCOG-LATER Study Group. Colorectal adenomas and cancers after childhood cancer treatment: a DCOG-LATER record linkage study. J Natl Cancer Inst 2018;110:758–67. [DOI] [PubMed] [Google Scholar]
- 8.Nathan PC, Ness KK, Hudson MM, et al. Cancer screening in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study (CCSS). J Clin Oncol 2009;27(Suppl):CRA6501. [Google Scholar]
- 9.Hodgson DC, Cotton C, Crystal P, et al. Impact of early breast cancer screening on mortality among young survivors of childhood Hodgkin’s lymphoma. J Natl Cancer Inst 2016;108. doi: 10.1093/jnci/djw010. [DOI] [PubMed] [Google Scholar]
- 10.Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 5.0. Children’s Oncology Group; 2018:1–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulder RL, Hudson MM, Bhatia S, et al. Updated breast cancer surveillance recommendations for female survivors of childhood, adolescent, and young adult cancer from the International Guideline Harmonization Group. J Clin Oncol 2020;38:4194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong FL, Bhatia S, Landier W, et al. Cost-effectiveness of the children’s oncology group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med 2014;160:672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeazel MW, Oeffinger KC, Gurney JG, et al. The cancer screening practices of adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer 2004;100:631–40. [DOI] [PubMed] [Google Scholar]
- 14.Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med 2010;153:442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA 2009;301:404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel CL, Kohler CL, Stratton KL, et al. Predictors of colorectal cancer surveillance among survivors of childhood cancer treated with radiation: a report from the Childhood Cancer Survivor Study. Cancer 2015;121:1856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan AP, Chen Y, Henderson TO, et al. Adherence to surveillance for second malignant neoplasms and cardiac dysfunction in childhood cancer survivors: a Childhood Cancer Survivor Study. J Clin Oncol 2020;38:1711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casillas J, Oeffinger KC, Hudson MM, et al. Identifying predictors of longitudinal decline in the level of medical care received by adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Health Serv Res 2015;50:1021–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagramanov D, Sutradhar R, Lau C, et al. Impact of the model of long-term follow-up care on adherence to guideline-recommended surveillance among survivors of adolescent and young adult cancers. Cancer Med 2021;10:5078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan PC, Ford JS, Henderson TO, et al. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J Clin Oncol 2009;27:2363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan PC, Agha M, Pole JD, et al. Predictors of attendance at specialized survivor clinics in a population-based cohort of adult survivors of childhood cancer. J Cancer Surviv 2016;10:611–8. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg ML, Barr RD, DiMonte B, et al. Childhood cancer registries in Ontario, Canada: lessons learned from a comparison of two registries. Int J Cancer 2003;105:88–91. [DOI] [PubMed] [Google Scholar]
- 23.Williams JI, Young W. Appendix 1: A summary of studies on the quality of health care administrative databases in Canada. In: Goel V, Williams JI, Anderson GM, et al. The ICES Practice Atlas, 2nd Edition — Patterns of Health Care in Ontario. Ottawa: Canadian Medical Association; 1996:339–45. [Google Scholar]
- 24.Low income cut-offs for 2005 and low income measures for 2004. Cat. no. 75F0002MIE — No. 004. Ottawa: Statistics Canada, 2006. [Google Scholar]
- 25.Dossa F, Metcalfe K, Sutradhar R, et al. Building the What Comes Next Cohort for BRCA1 and BRCA2 testing: a descriptive analysis. CMAJ Open 2021;9:E874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baliunas D, Selby P, de Oliveira C, et al. Primary care-based smoking cessation treatment and subsequent healthcare service utilisation: a matched cohort study of smokers using linked administrative healthcare data. Tob Control 2023;32:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paszat LF, Sutradhar R, Gu S, et al. Annual surveillance mammography after early-stage breast cancer and breast cancer mortality. Curr Oncol 2016;23:e538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Li Q, Kassam A, et al. Specialty palliative care and symptom severity and control in adolescents and young adults with cancer. JAMA Netw Open 2023;6:e2338699. doi: 10.1001/jamanetworkopen.2023.38699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner JP, Starfield BH, Steinwachs DM, et al. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care 1991;29:452–72. [DOI] [PubMed] [Google Scholar]
- 30.Weiner JP, Abrams C, Bodycombe D. The Johns Hopkins ACG case-mix system. Version 6.0 release notes. Baltimore: Johns Hopkins Bloomberg School of Public Health; 2003. [Google Scholar]
- 31.The Johns Hopkins ACG System: excerpt from technical reference guide. Baltimore: John Hopkins University; 2009. [Google Scholar]
- 32.Wong FL, Lee JM, Leisenring WM, et al. Health benefits and cost effectiveness of children’s oncology group breast cancer screening guidelines for chest-irradiated Hodgkin lymphoma survivors. J Clin Oncol 2023;41:1046–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruitt SL, Shim MJ, Mullen PD, et al. Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: a systematic review. Cancer Epidemiol Biomarkers Prev 2009;18:2579–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ioannou GN, Chapko MK, Dominitz JA. Predictors of colorectal cancer screening participation in the United States. Am J Gastroenterol 2003;98:2082–91. [DOI] [PubMed] [Google Scholar]
- 35.Yan AP, Chen Y, Henderson TO, et al. Adherence to surveillance for second malignant neoplasms and cardiac dysfunction in childhood cancer survivors: a childhood cancer survivor study. J Clin Oncol 2020;38:1711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz SJ, Hofer TP. Socioeconomic disparities in preventive care persist despite universal coverage: breast and cervical cancer screening in Ontario and the United States. JAMA 1994;272:530–4. [PubMed] [Google Scholar]
- 37.Lofters AK, Kopp A, Vahabi M, et al. Understanding those overdue for cancer screening by five years or more: a retrospective cohort study in Ontario, Canada. Prev Med 2019;129:105816. [DOI] [PubMed] [Google Scholar]
- 38.Cherven B, Mertens A, Meacham LR, et al. Knowledge and risk perception of late effects among childhood cancer survivors and parents before and after visiting a childhood cancer survivor clinic. J Pediatr Oncol Nurs 2014;31:339–49. [DOI] [PubMed] [Google Scholar]
- 39.Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors’ knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002;287:1832–9. [DOI] [PubMed] [Google Scholar]
- 40.Henderson TO, Friedman DL, Meadows AT. Childhood cancer survivors: transition to adult-focused risk-based care. Pediatrics 2010;126:129–36. [DOI] [PubMed] [Google Scholar]
- 41.Nathan PC, Daugherty CK, Wroblewski KE, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv 2013;7:275–82. [DOI] [PubMed] [Google Scholar]
- 42.Eshelman-Kent D, Kinahan KE, Hobbie W, et al. Cancer survivorship practices, services, and delivery: a report from the Children’s Oncology Group (COG) nursing discipline, adolescent/young adult, and late effects committees. J Cancer Surviv 2011;5:345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh H, Bernstein CN, Samadder JN, et al. Screening rates for colorectal cancer in Canada: a cross-sectional study. CMAJ Open 2015;3:E149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruffin MT, Gorenflo DW, Woodman B. Predictors of screening for breast, cervical, colorectal, and prostatic cancer among community-based primary care practices. J Am Board Fam Pract 2000;13:1–10. [DOI] [PubMed] [Google Scholar]
- 45.Richard A, Rohrmann S, Schmid SM, et al. Lifestyle and health-related predictors of cervical cancer screening attendance in a Swiss population-based study. Cancer Epidemiol 2015;39:870–6. [DOI] [PubMed] [Google Scholar]
- 46.Wools A, Dapper E, de Leeuw JRJ. Colorectal cancer screening participation: a systematic review. Eur J Public Health 2016;26:158–68. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian S, Klosterman M, Amonkar MM, et al. Adherence with colorectal cancer screening guidelines: a review. Prev Med 2004;38:536–50. [DOI] [PubMed] [Google Scholar]
- 48.Daskalakis C, DiCarlo M, Hegarty S, et al. Predictors of overall and test-specific colorectal cancer screening adherence. Prev Med 2020. Feb. 8 [Epub ahead of print];133:106022. doi: 10.1016/j.ypmed.2020.106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bujanda L, Sarasqueta C, Zubiaurre L, et al. EPICOLON Group. Low adherence to colonoscopy in the screening of first-degree relatives of patients with colorectal cancer. Gut 2007;56:1714–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med 2005;20:989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones RM, Devers KJ, Kuzel AJ, et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med 2010;38: 508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quintero E, Castells A, Bujanda L, et al. COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012;366:697–706. [DOI] [PubMed] [Google Scholar]
- 53.Hobbie WL, Stuber M, Meeske K, et al. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. J Clin Oncol 2000;18:4060–6. [DOI] [PubMed] [Google Scholar]
- 54.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]