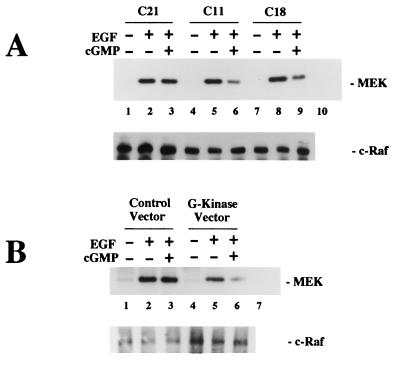
FIG. 8.
Effect of 8-pCPT-cGMP on EGF-induced c-Raf kinase activation measuring MEK phosphorylation. Cells were cultured as described for Fig. 5, and cells were extracted in the presence of phosphatase and protease inhibitors. c-Raf kinase was immunoprecipitated from the extracts, and washed immunoprecipitates were incubated with MEK-1 and [γ-32PO4]ATP for 10 min to determine Raf kinase activity as described in Materials and Methods. Control immunoprecipitates obtained with nonimmune rabbit serum were incubated with MEK-1 in parallel reactions (lane 10 in panel A and lane 7 in panel B). Samples were split in half and analyzed by SDS-PAGE, with proteins of one of the gels electroblotted onto membranes. Phosphorylated MEK-1 was visualized by autoradiography (upper panels); incubation of the blot with a c-Raf antibody demonstrated similar amounts of c-Raf kinase present in the immunoprecipitates (lower panels). (A) Individual clones of stably transfected BHK cells (approximately 107 cells) expressing very low G-kinase activity (C21) or expressing significant amounts of G-kinase activity (C11 and C18) were analyzed as described above. MEK-1 phosphorylation by c-Raf kinase immunoprecipitated from untreated cells was detectable on longer exposures (not shown). (B) Approximately 106 wild-type BHK cells were transiently transfected with either control vector or G-kinase vector as described for Fig. 5C and analyzed as described above.