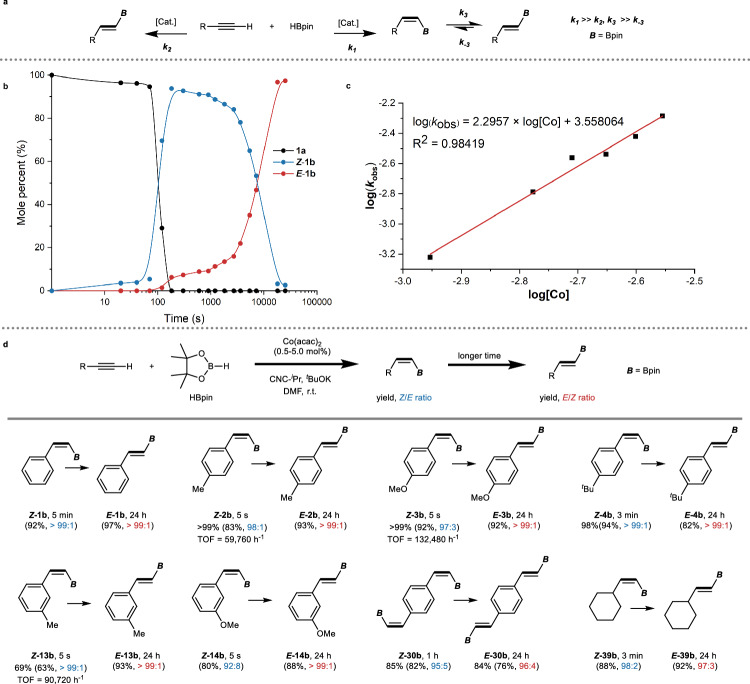
Fig. 4. Kinetic investigation.
a Proposed equilibrium equation of the hydroboration of terminal alkynes. b Kinetic profile of the hydroboration of phenylacetylene (1a). Reaction conditions: 1a (1.0 equiv, 0.4 mmol), HBpin (2.0 equiv), Co(acac)2 ([Co], 0.5 mol%), CNC-iPr (1.4 equiv to [Co]), tBuOK (5.6 equiv to [Co]) in DMF (0.5 ml) at room temperature (r.t.). c Kinetic analysis of the formal reaction order based on the concentration of catalyst ([Co]). d Time-dependent stereoselective hydroboration of terminal alkynes. Reaction conditions: terminal alkyne (1.0 equiv, 0.4 mmol), HBpin (3.0 equiv), Co(acac)2 ([Co], 0.5–5 mol%), CNC-iPr (1.4 equiv to [Co]), tBuOK (5.6 equiv to [Co]) in DMF (0.5 ml) at room temperature (r.t.). See Supplementary Information for experimental details. 1H NMR yields are shown with methylene bromide as the internal standard. Isolated yields and Z/E ratios in parenthesis. The reaction time for the transformation of the E isomers was not optimized.