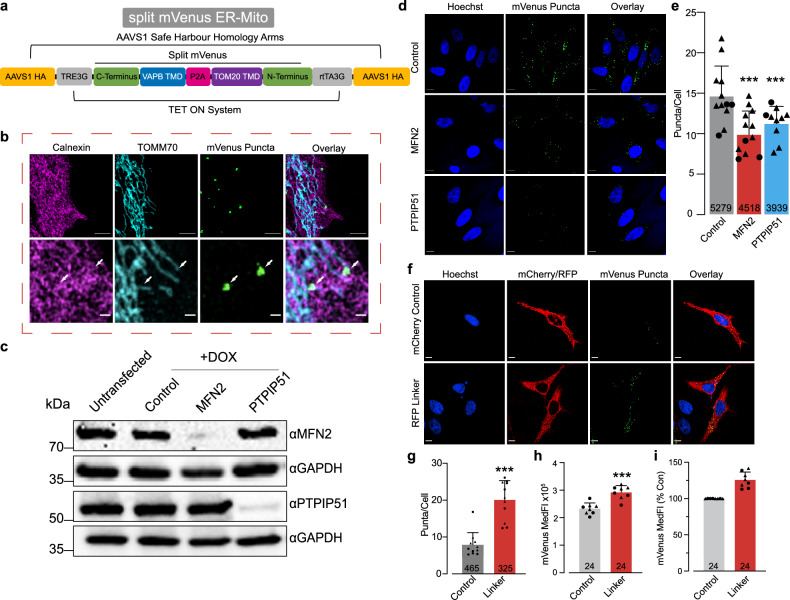
Fig. 1. Functional characterisation of an ER-Mito reporter cell line.
a A scheme of the split mVenus ER-Mito construct used to generate ER-Mito reporter cell lines. The split mVenus system is composed of the transmembrane domains of TOMM20 and VAPB fused to the N- and C-terminal domains of nonfluorescent protein fragments of mVenus, respectively. These constructs were combined within the AAVS1 safe harbour site of the HeLa Cas9 cell line under the control of a promoter regulated by tetracycline. b Partial co-localisation of the split mVenus ER-Mito reporter with mitochondria and the ER assessed by immunostaining and N-SIM. Mitochondria were stained with an anti-TOMM70 antibody, and the ER was stained with an anti-Calnexin antibody. Scale bar, 5 μm. c Analysis of the downregulation of MFN2 or PTPIP51 in ER-Mito reporter cell lines. ER-Mito reporter cells were transfected with either non-targeting (control), MFN2 or PTPIP51 siRNA for four days and treated with 1 μg/mL doxycycline for 24 h. Cell lysates were analysed by western blotting using the indicated antibodies. d, e The siRNA-mediated downregulation of MFN2 or PTPIP51 decreases the number of mVenus puncta. ER-Mito reporter cells were transfected with non-targeting (control), MFN2 and PTPIP51 siRNA for 4 days and treated with 1 μg/mL doxycycline for 24 h. Representative images of the mVenus puncta (d) detected by spinning disc confocal microscopy in cells co-stained with Hoechst to detect the nuclei and quantified in (e). Scale bar 10 μm. Mean ± S.D, number indicates number of cells analysed, n = 4 (1× Clone 1, 3× Clone 2), data points = mVenus puncta/cell averaged per coverslip (Con = 12, MFN2 = 12, PTPIP51 = 10), (circles = Clone 1, triangles = Clone 2), data analysed using mixed effects models with significance tests performed using Satterthwaite’s degrees of freedom method with ImerTest. f, g Overexpression of an artificial RFP linker (composed of N-terminal of mitochondrial protein mAKAP1 and C-terminal, ER localisation sequence of yUBC6 bridged by an RFP (mAKAP1 [34–63]-mRFP-yUBC6) [68]) increases the number of mVenus puncta in ER-Mito reporter cells. Cells were transfected with the RFP Linker or a control-plasmid expressing mito-mCherry (N-terminal of mitochondrial protein mAKAP1 fused to mCherry without the ER localisation sequence) and treated with 1 μg/mL doxycycline for 24 h + 48 h in medium without doxycycline. Representative images (f) of mVenus puncta detected by spinning disc confocal microscopy in cells co-stained with Hoechst and quantified in (g). Scale bar 10 μm. Mean ± S.D, number indicates number of cells analysed, n = 4 (2× Clone 1, 2× Clone 2), data points = mVenus puncta/cell averaged per coverslip (Con = 11, Linker = 11), (circles = Clone 1, triangles = Clone 2), mixed effects models with significance tests performed using Satterthwaite’s degrees of freedom method with ImerTest. h, i Overexpression of the ER-mito linker increases mVenus fluorescence intensity. ER-Mito reporter cells were transfected with ER-Mito linker (mAKAP1 [34–63]-mRFP-yUBC6) [68] and treated with 1 μg/mL doxycycline for 24 h + 48 h in medium without doxycycline. The MedFI was measured by flow cytometry (h) in both clones, and the percentage change in MedFI is shown in (i). Mean ± S.D., n = 8 (4× Clone 1, 4× Clone 2), data points = mVenus medFI averaged per biological replicate (Con = 8, Linker = 8), (circles = Clone 1, triangles = Clone 2) mixed effects models with significance tests performed using Satterthwaite’s degrees of freedom method with ImerTest.