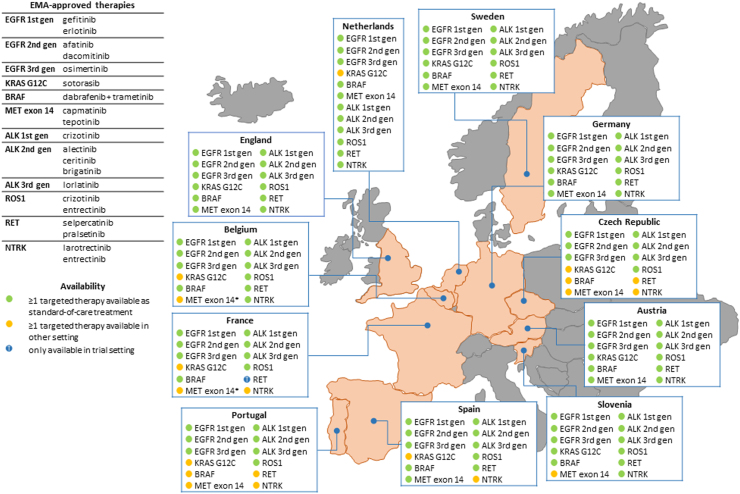
Fig. 2.
Availability of targeted therapies for patients with metastatic NSCLC across eleven European countries. For each country, the displayed information was provided by a detailed questionnaire (Supplementary File 1) that was completed by a (molecular) pathologist, clinical scientist in molecular pathology and/or (pulmonary) oncologist with expertise in the field of NSCLC. ∗Presented availability concerns crizotinib (FDA-approved), due to its better availability compared to tepotinib and capmatinib (EMA-approved) (see also Table 2).