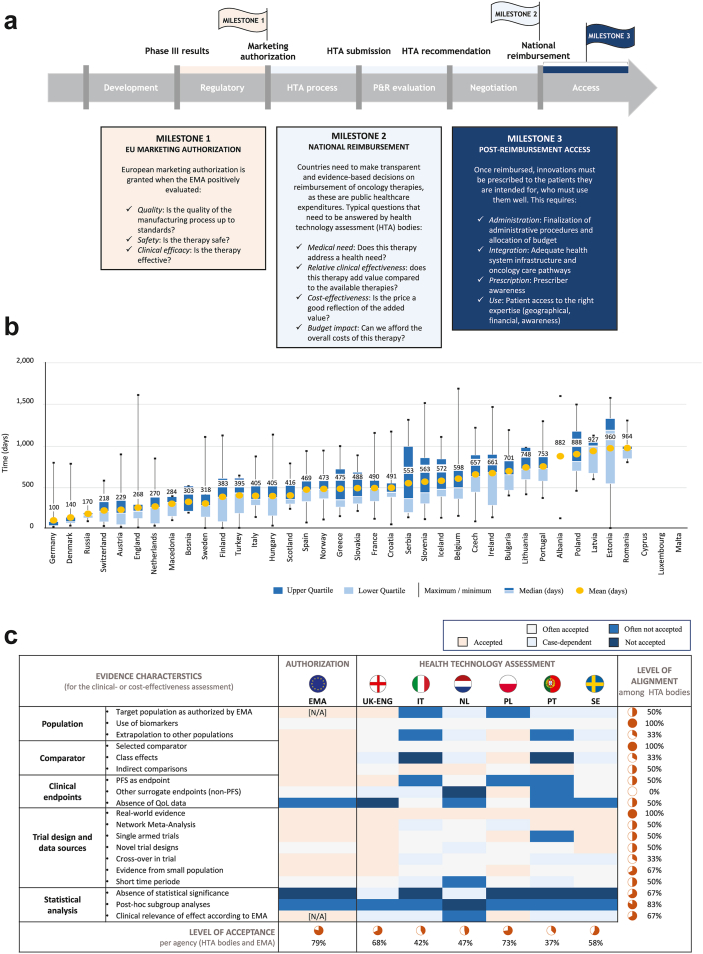
Fig. 2.
Inequalities in patient access in Europe: (a) the path for novel therapies, (b) assessing delays, and (c) evidence requirements for patient access.
(a) Thee milestones must be achieved before patients have access to new therapies. (b) The median time to availability in days in European countries (2017–2020), assessed from the date of marketing authorization to, for most countries, the date of acceptance on the reimbursement list. (c) Different evidence requirements are used across Europe, delaying the time to medicine access (Figure 2a adapted and modified from the European Federation of Pharmaceutical Industries and Associations (EFPIA), figure 2b adapted and modified from IQVIA, and figure 2c adapted from EFPIA67, 83). Abbreviations: EU, European Union; EMA, European Medicines Agency; HTA, health technology assessment; P&R, pricing and reimbursement; UK-ENG, United Kingdom-England; IT, Italy; NL, the Netherlands; PL, Poland; PT, Portugal; SE, Sweden; PFS, progression-free survival; QoL, quality of life.