Extended Fig. 7. Extended biochemical data for the 5-ASA metabolizing thiolase enzymes.
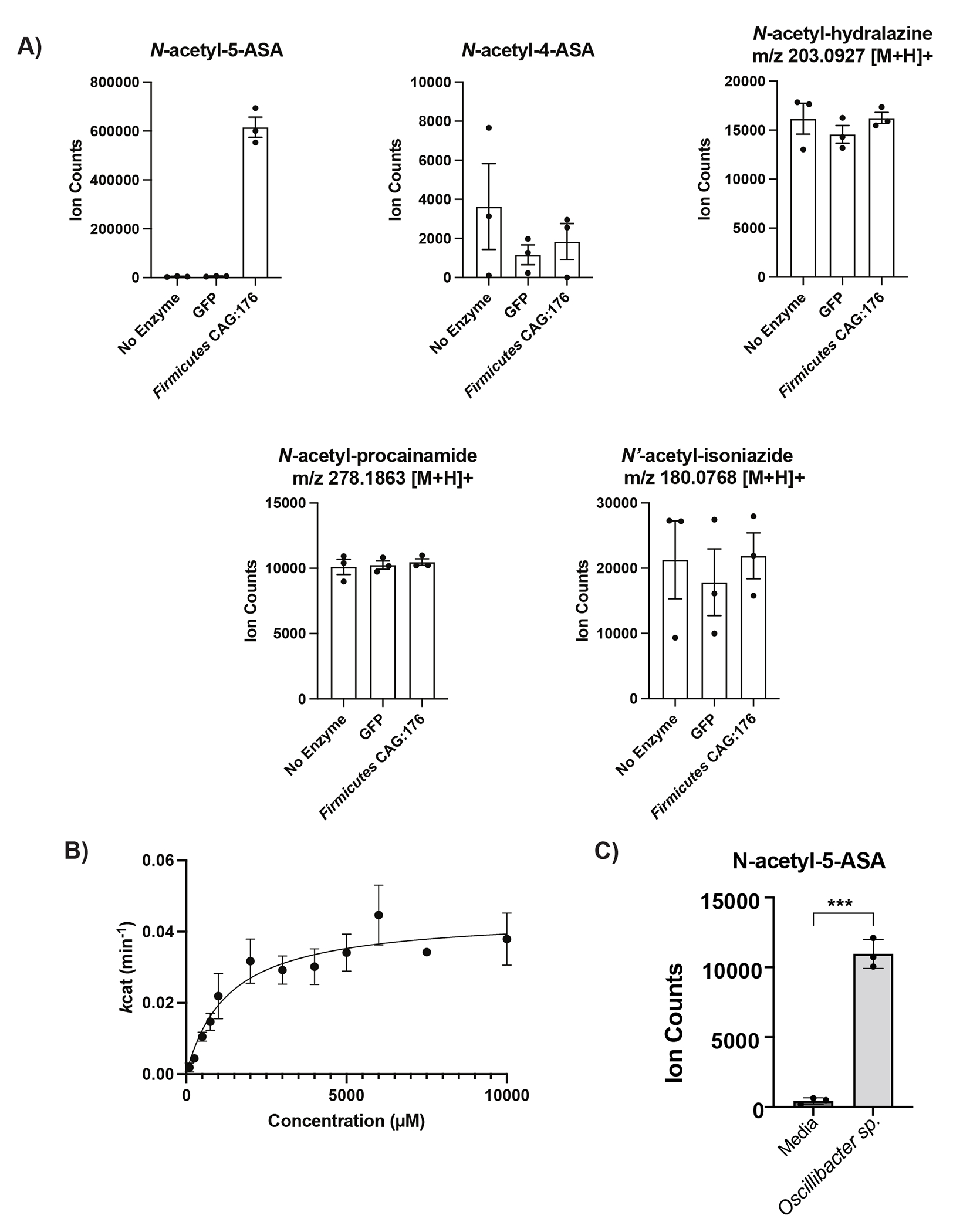
(A) In vitro competition assay with 1 mM of 5-ASA, 4-ASA, procainamide, hydralazine and isoniazide demonstrates relative specificity of the Firmicutes CAG:176 thiolase (FcTHL) for 5-ASA. N=3 biologically independent samples per enzyme/condition. (B) Shown is a representative Michaelis-Menten plot of n=1 thiolase enzyme preparation, conducted in technical triplicates at each concentration of 5-ASA; summary data is from n=5 biologically independent experiments each conducted in technical triplicate. (C) Live culture of Oscillibacter sp., strain KLE 1745 encoding another predicted thiolase gene (UniRef90 R6TIX3) was capable of acetylation of 5-ASA to N-acetyl 5-ASA. N=3 biologically independent samples per condition, representative data from n=2 independent experiments, unpaired, two-sided T-test, *** = p = 0.0015. Data are presented as mean values +/− SEM.
