Figure 4: Heterologous expression and purification of a gut microbial acetyltransferase confirms 5-ASA acetylation activity in vitro.
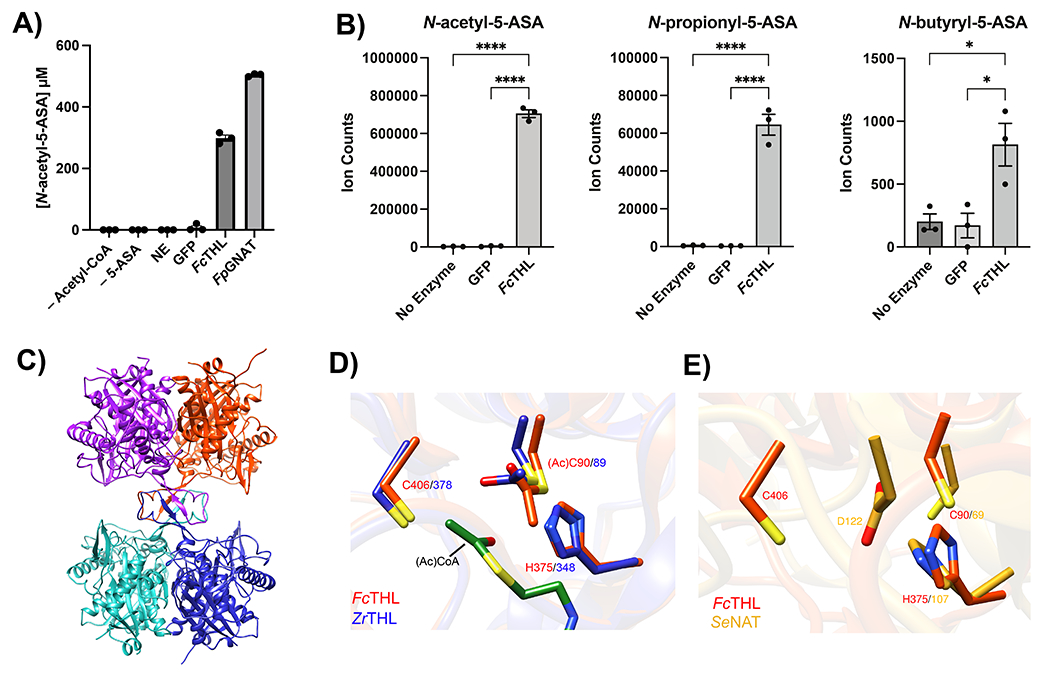
(A) A predicted thiolase (R6CZ24) from an uncultured Firmicutes (FcTHL) and a predicted acyl CoA N-acyltransferase (C7H1G6) from F. prausnitzii (FpGNAT) convert 5-ASA to N-acetyl-5-ASA in the presence of acetyl-CoA. Reactions were carried out for 6 hr at 37°C with 1 mM of each substrate and 50 μM enzyme. N=3 biologically independent samples per enzyme/condition. (B) An in vitro pooled acyl-CoA competition assay with 1 mM of each acyl-CoA species, confirms varying length acyl groups can be donated to 5-ASA, as detected in our analysis in patients with IBD (Fig 2c). N=3 biologically independent samples per enzyme/condition. (****=p<0.0001, *=p= 0.02, one-way ANOVA followed by Tukey’s multiple comparisons test.) (C) Tetrameric crystal structure of FcTHL, shown as a ribbon diagram with one dimer in purple and orange and the other dimer in green and blue. Two tightly interacted dimers form a tetramer through an L-domain. D). Alignment of an acetylated monomer from the FcTHL with an acetylated biosynthetic thiolase monomer (1DM3) in complex with acetyl CoA from Zoogloea ramigera (ZrTHL) highlights good agreement and reveals an overlapping Cys-His-Cys catalytic triad poised to acetylate a substrate. (E) Using a similar method of protein structure superimposition, the crystallized Salmonella enterica typhimurium NAT (PDB ID: 1E2T, SeNAT, known to acetylate 5-ASA) and the Firmicutes thiolase have very different overall structures, yet both enzymes’ active sites contain cysteine and histidine residues in similar positions and perform similar reactions (Extended Fig11a-b). Where applicable, data are presented as mean values +/− SEM.
