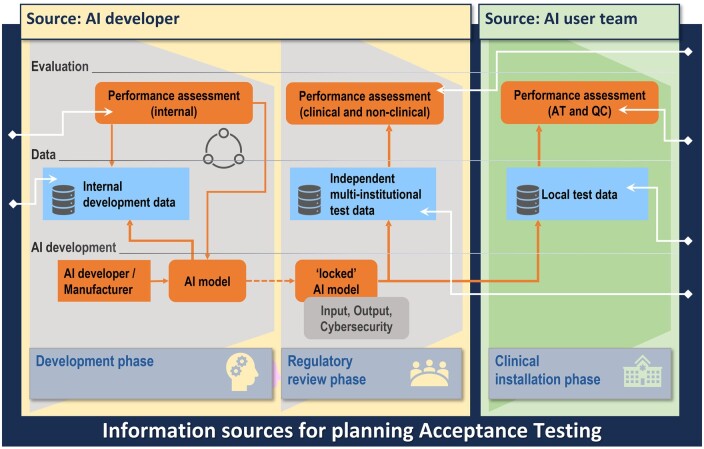
Figure 2.
Overview of the different information sources involved in AI development, regulatory review, and clinical installation. Upon model completion (left), the locked model either undergoes regulatory review, additional retrospective or prospective multi-institutional validation (middle), or local clinical installation (right). Finally, before the tool is deployed for clinical use, testing with a site-specific, locally curated test set, the composition of which could be facilitated by vendor transparency, is essential.