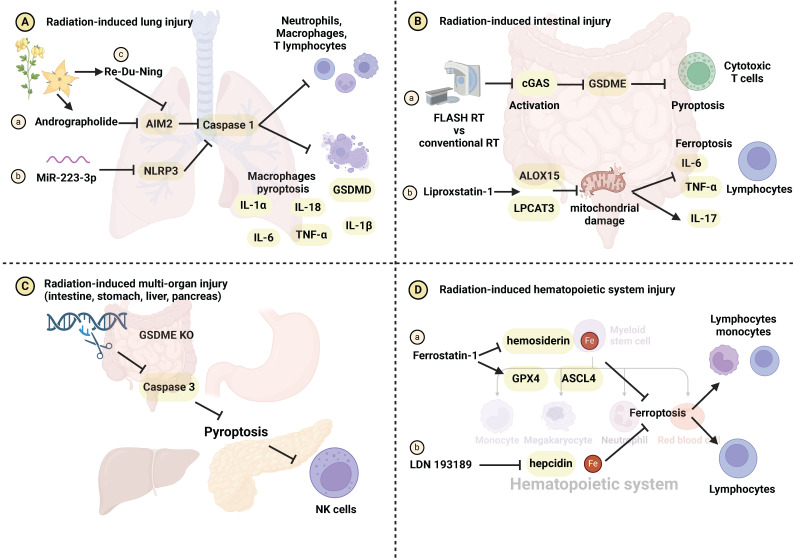
Figure 3.
The role of immune regulation in pyroptosis- and ferroptosis-related radiation damage. (A) Radiation-induced lung injury. (Aa-b) Andrographolide and miR-223-3p inhibit macrophage pyroptosis, the release of IL-1α, IL-1β, IL-6, IL-18, TNF-α and radiation-induced lung injury through the AIM2/caspase-1 and NLRP3/caspase-1 axes, respectively. (Ac) RDN inhibits the AIM2/caspase-1 axis, reducing the recruitment of immune cells such as macrophages, neutrophils, and T lymphocytes, and mitigating radiation-induced lung injury. (B) Radiation-induced intestinal injury. (Ba) FLASH-RT inhibits the cGAS-STING pathway, attenuating GSDME-induced pyroptosis and cytotoxic T cells infiltration, ultimately protecting intestinal cells from radiation damage. (Bb) Liproxstatin-1 upregulates LPCAT3 and ALOX15 expression and inhibits mitochondrial damage, ultimately preventing ferroptosis, the release of IL-6, IL-17, TNF-α and lymphocytes infiltration. (C) Radiation-induced multi-organ (intestine, stomach, liver and pancreas) injury. Inhibition of pyroptosis by regulating the GSDME/caspase-3 axis blocks NK cells recruitment, mitigating radiation-induced multi-organ damage. (D) Radiation-induced hematopoietic system injury. (Da-b) Ferrostatin-1 and LDN 193189 inhibit iron metabolism to protect the hematopoietic system from radiation damage.