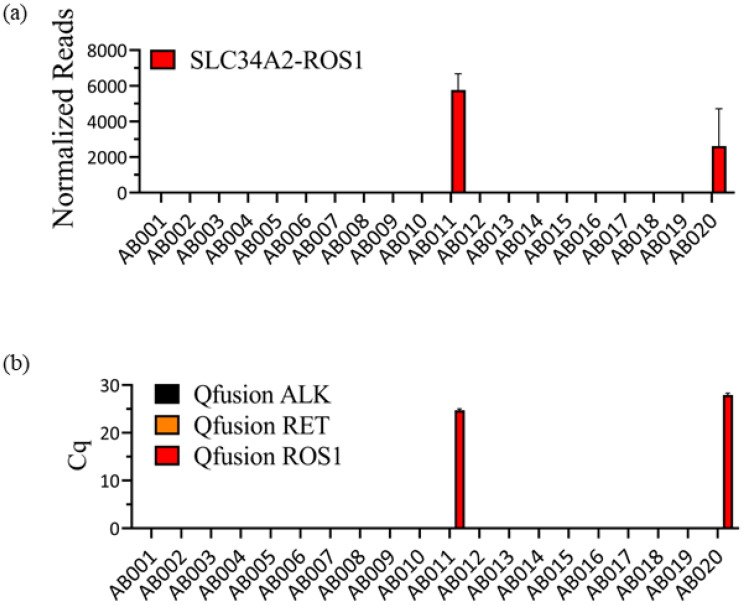
Figure 3.
Consistency between OptiSeqTM lung cancer fusion NGS panel and QfusionTM ALK, RET, or ROS1 fusion detection assay results. (a) Analysis of twenty FFPE samples using the OptiSeqTM lung cancer fusion NGS panel revealed the presence of SLC34A2-ROS1 fusion in two patient samples. The Y-axis represents normalized reads, and the error bars indicate the standard deviations of three replicates. (b) The same twenty FFPE samples were subjected to analysis using QfusionTM assays. The results confirmed ROS1 fusion positivity in two patients with SLC34A2-ROS1 fusion. QfusionTM ALK and RET fusion detection assays showed no ALK and RET fusions. The error bars depict the standard deviations of three replicates.