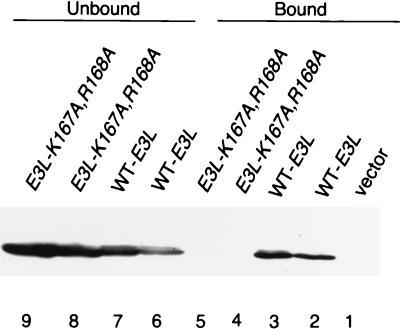
FIG. 3.
The K167A and R168A mutations impair dsRNA binding by E3 in vitro. Transformants of strain J82 containing the indicated HA-tagged E3L alleles or vector alone (p1079) were grown in SD medium for ∼30 h and then shifted to inducing conditions (SGal medium) for ∼12 h, and whole-cell extracts were prepared. Aliquots containing 100 μg of total protein were incubated with poly(I-C)-agarose for 1 h at 4°C. Proteins which bound to poly(I-C)-agarose were collected by centrifugation and eluted by boiling in 2× Laemmli sample buffer. Unbound proteins in the supernatant were trichloroacetic acid precipitated, washed with ethanol, and resuspended by boiling in 2× Laemmli sample buffer. Proteins were resolved by SDS-PAGE using 8 to 16% gradient gels and subjected to immunoblot analysis using monoclonal antibodies against the HA epitope (HA-12CA5) and ECL to detect immune complexes. Lanes 1 to 5 contain the fraction of E3 proteins which bound to poly(I-C)-agarose; lanes 6 to 9 contain the fraction of E3 proteins which did not bind to poly(I-C)-agarose.