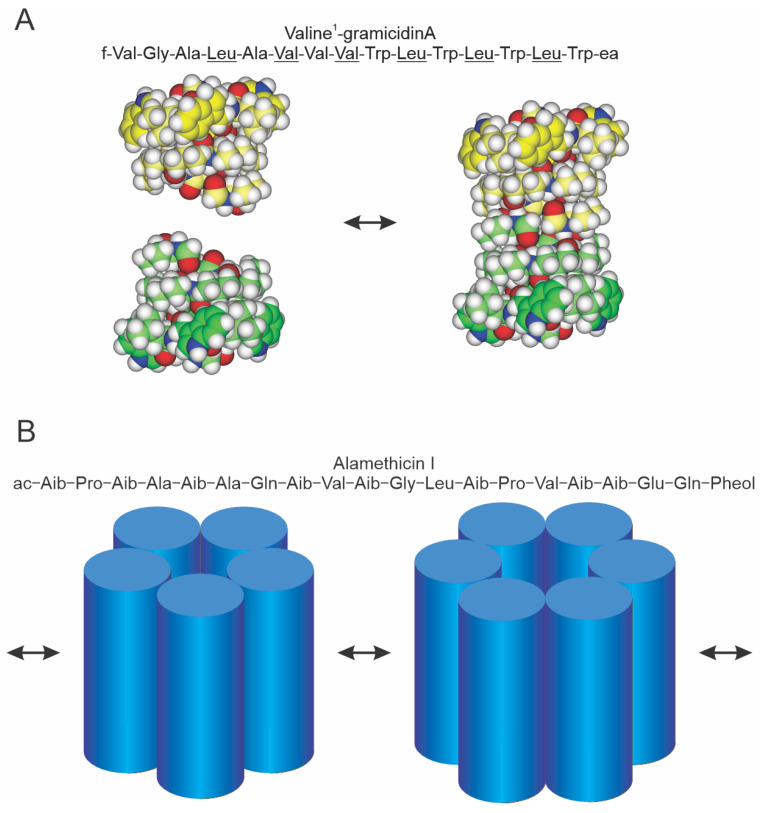
Figure 1.
Schematic models of gramicidin and alamethicin channels. (A) Top: sequence of [Val1]gA [34], the major gramicidin species in naturally occurring mixture of peptides [35]; f is formyl, ea ethanolamine and the D-amino acids are underlined. Bottom: gramicidin channels form and disappear, as indicated by the arrows, by a transmembrane association/dissociation [36]. Left, atomic resolution structures of the β6.3-helical monomers, the two subunits are depicted some distance apart; right, atomic resolution structure of the β6.3-helical conducting dimer. The carbons in the two subunits are colored green and yellow, respectively, with the carbon atoms in the Trp side chains emphasized. Blue is nitrogen, red is oxygen and white is hydrogen. (B) Top: sequence of alamethicin I [37], the major species of alamethicin; ac is acetate, Aib α-isobutyric acid and Pheol phenylalcohol. Bottom: different interconverting oligomeric states, as indicated by the arrows, of the bilayer-spanning channel. The number of subunits may change by the association/dissociation of bilayer-spanning subunits or oligomers or by the accretion of subunits at the bilayer/solution interface that inserts into the bilayer [33,38].