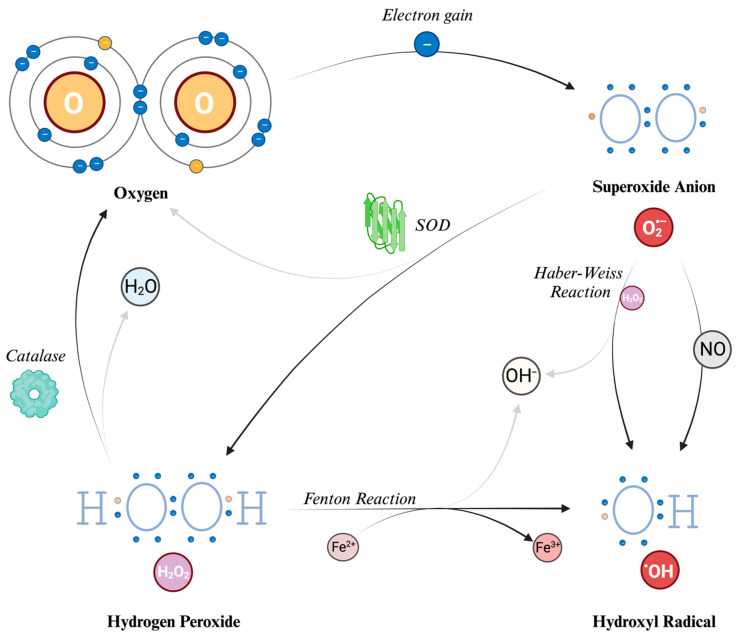
Figure 2.
Reactive Oxygen Species (ROS): their structure and formation. The process begins with molecular oxygen (O2), which has two unpaired electrons in the outer orbital. Thus, it can acquire an electron to form the superoxide anion (O2−), which is a radical (i.e., with an unpaired electron). Superoxide dismutase (SOD) converts superoxide into hydrogen peroxide (H2O2) and oxygen. Catalases can further break down H2O2 into water and oxygen. The Haber–Weiss reaction generates hydroxyl radicals (HO•) and hydroxyl ions (OH−) from superoxide and H2O2. The Fenton reaction also produces hydroxyl radicals and hydroxyl ions by reducing H2O2 in the presence of iron ions. O: oxygen atom. H: hydrogen atom. H2O: water molecule. O2−: superoxide anion. H2O2: hydrogen peroxide. HO•: hydroxyl radical. OH−: hydroxyl ion. NO: nitric oxide. Fe2+: iron in reduced state. Fe3+: iron in oxidized state. SOD: superoxide dismutase.