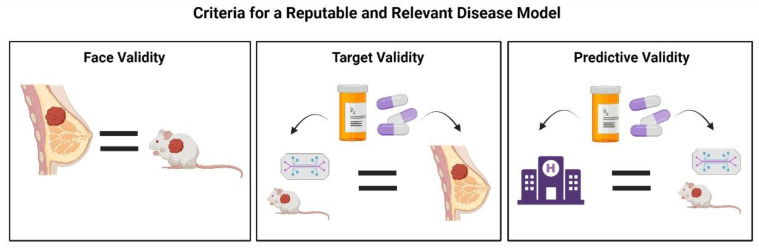
Figure 3.
Breast cancer preclinical models must meet three criteria to be considered both reputable and relevant. The translatability of preclinical models plays a decisive role in drug attrition and success rates. For data retrieved from preclinical studies to reflect clinical outcomes, it is important that in vitro disease models meet three criteria: face validity, target validity, and predictive validity. Face validity refers to the biological similarities between the preclinical model and the disease itself. Target validity is attained when drug responses are similar in both the model and clinical setting. Finally, predictive validity is determined based on similar responses observed in both the model and clinical settings. Created with Biorender.com (accessed on 11 January 2024).