Figure 1.
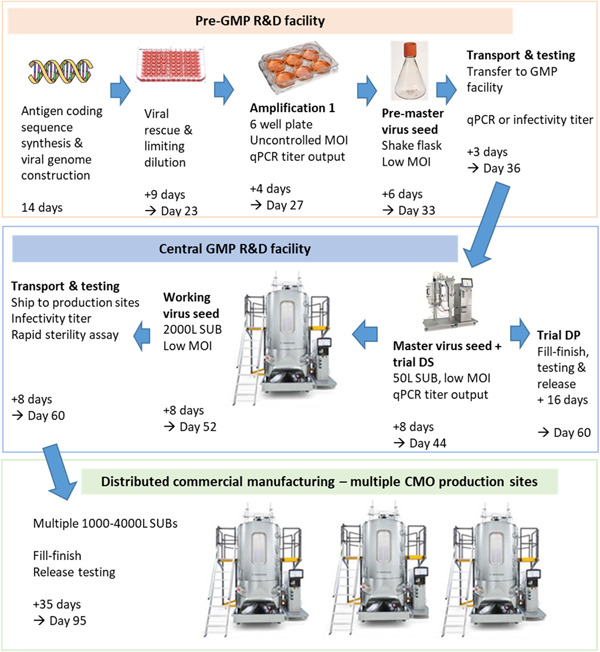
Rapid virus seed production enables early vaccine release. Detailed scheme for virus seed production, including anticipated timing of each step. This timing assumes the availability of facilities, equipment, materials, and staff. It further assumes cell expansion for MVS, WVS, and DS production occurring in parallel with preceding steps of virus seed generation, potentially making use of a “bleed and dilute” strategy to hold cells at 50–200 L volume and hence ensure immediate readiness for WVS/DS production upon seed availability. Finally, it assumes decisions to proceed “at financial risk” at all stages, ahead of the availability of any test results other than those stated, but with the completion of full testing before drug product release. CMO, contract manufacturing organization; DP, drug product; DS, drug substance; GMP, Good Manufacturing Practice; MOI, multiplicity of infection; MVS, master virus seed; qPCR, quantitative polymerase chain reaction; R&D, research and development; SUB, single‐use bioreactor; USP, upstream process; VPs, viral particles; WVS, working virus seed.
