Abstract
Background:
Long-term use of levodopa for Parkinson’s disease (PD) treatment is often hindered by development of motor complications including levodopa-induced dyskinesia (LID). The substantia nigra pars reticulata (SNr) and globus pallidus internal segment (GPi) are the output nuclei of the basal ganglia. Dysregulation of SNr and GPi activity contributes to PD pathophysiology and LID.
Objectives:
Determine whether direct modulation of SNr GABAergic neurons and SNr projections to the pedunculopontine nucleus (PPN) regulates PD symptoms and LID in a mouse model.
Methods:
We expressed Cre-recombinase activated channelrhodopsin-2 (ChR2) or halorhodopsin (NpHR) AAV2 vectors selectively in SNr GABAergic neurons of Vgat-IRES-Cre mice in a 6-hydroxydopamine model of PD to investigate whether direct optogenetic modulation of SNr neurons or their projections to the PPN regulates PD symptoms and LID expression. The forepaw stepping task, mouse LID rating scale, and open field locomotion were used to assess akinesia and LID, respectively, to test the effect of SNr modulation.
Results:
Akinesia was improved by suppressing SNr neuron activity with NpHR. LID was significantly reduced by increasing SNr neuronal activity with ChR2, which did not interfere with the anti-akinetic effect of levodopa. Optical stimulation of ChR2 in SNr projections to the PPN recapitulated direct SNr stimulation.
Conclusions:
Modulation of SNr GABAergic neurons alters akinesia and LID expression in a manner consistent with the rate model of basal ganglia circuitry. Moreover, the projections from SNr to PPN likely mediate the antidyskinetic effect of increasing SNr neuronal activity, identifying a potential novel role for the PPN in LID.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder with prominent motor symptomatology including akinesia, rigidity, resting tremor, and postural instability. Pathologically, progressive degeneration of nigrostriatal dopaminergic neurons and projections resulting in loss of striatal dopamine (DA) produces motor symptoms.1–4 Pharmacotherapy with L-DOPA dramatically alleviates motor symptoms; however, motor complications including abnormal involuntary movements (AIMs) termed L-DOPA-induced dyskinesia (LID) are noted in up to 80% of patients within 5 years of treatment.5,6 The exact mechanisms underlying the development and expression of PD symptoms and LID are still not fully understood, but aberrant basal ganglia plasticity likely develops from DA denervation in combination with DA replacement therapy.7,8 The basal ganglia integrates cortical, thalamic, and brainstem inputs to modulate motor circuits via the primary output nuclei, the internal segment of the globus pallidus (GPi) and substantia nigra pars reticulata (SNr).9,10 Modulation of basal ganglia output by surgical lesions or high-frequency deep brain stimulation (DBS) of the GPi or subthalamic nucleus (STN) are effective neurosurgical interventions for alleviating PD symptoms and LID,11 though the precise mechanisms that mediate DBS effects remain debated.12
Based on the classic model of basal ganglia connectivity and function, the predicted effect of DA loss in PD is to increase the firing rate of GABAergic SNr projection neurons which should inhibit thalamic motor output to disallow movement,13,14 though SNr neuronal activity has been reported to be increased,15,16 decreased,17 or unchanged by dopamine depletion in animal models.17–20 Therapeutic doses of L-DOPA are thought to reduce SNr firing to facilitate movement, and during LID these neurons are predicted to be excessively inhibited due to sensitized response to DA.21,22 However, the clinical efficacy of pallidotomy or thalamotomy in reducing LID contradicts this notion,23 though most studies show lower SNr firing rates with L-DOPA treatment.24,25 Few attempts at modulating the SNr/GPi in rodent models of LID have been reported,26 although excitotoxic lesion of these structures had no effect.27 The SNr is comprised of mostly GABAergic neurons but contains scattered dopaminergic, cholinergic,28 and glutamatergic neurons.29 A better understanding of the contribution of SNr GABAergic neurons to PD symptoms and LID, and their connectivity to downstream target nuclei may elucidate the neural mechanisms underlying dysregulated basal ganglia output in PD and LID.
In this manuscript, we assess the effects of directly modulating SNr GABAergic neurons on akinesia and LID in a mouse model using Cre-dependent expression of activating and inhibitory opsins in Vgat-IRES-Cre mice. We show that increasing SNr activity reduces LID expression, whereas decreasing SNr activity improves akinesia. Pedunculopontine nucleus (PPN) dysfunction has been widely reported in PD. As the selective upregulation of cholinergic30,31 and caudal glutamatergic32 PPN neuron activity can improve motor function in rodent PD models and PPN neurons show increased markers of activity following LID,33,34 we focused on SNr-to-PPN projections and identify the PPN as a SNr target that is sufficient to recapitulate the antidyskinetic effect of optogenetic activation of SNr neurons.
METHODS
Detailed methods are included in the supplementary information.
Mice
To express opsins in GABAergic neurons, we used male and female Vgat-IRES-Cre mice (Vgat-Cre; Slc32a1tm2(cre)Lowl/J, Jackson Laboratory)35 at 12–24 weeks of age. Animal use followed the National Institutes of Health guidelines and was approved by the Institutional Animal Care and Use Committee of New York University.
6-OHDA lesion, AAV injection and optic fiber implantation
Surgery.
Mice were anesthetized with isoflurane and head-fixed in a stereotaxic frame. All procedures were conducted during the same surgical session.
Dopaminergic lesions.
We infused 6-hydroxydopamine (6-OHDA; 4.5 μg) targeting the left medial forebrain bundle (MFB), with desipramine (25 mg/kg IP) given 30 minutes before 6-OHDA infusion.36
Virus injection and optical fiber implantation.
100–200 nl of viral stocks (UNC Vector Core) of Cre-inducible AAV vectors encoding channel rhodopsin (ChR2; AAV2-EF1a-DIO-hChR2(H134R)-EYFP), halorhodopsin (NpHR; AAV2-EF1a-DIO-eNpHR3.0-EYFP), or eYFP (AAV2-Ef1a-DIO-EYFP) were injected into the ipsilesional SNr. Optic fibers (diameter: 200 μm; Thorlabs) were placed above the injected SNr or corresponding PPN. After 3–4 weeks of intensive postoperative care, we evaluated limb use asymmetry with a supported forepaw treadmill stepping task.36 Only mice exhibiting < 30% steps taken with the contralesional paw were kept in the study. In total, 33 mice received 6-OHDA lesions, viral injection, and optic fiber implantation. Of these, 4 were excluded for incorrect fiber placement and 3 for poor virus expression verified by histology at the end of the study. Thus, 79% mice were retained in the final analysis.
L-DOPA treatment and Optogenetic stimulation
L-DOPA methyl ester (3 mg/kg) and benserazide (12.5 mg/kg) were given together by IP injection. LID was primed by giving L-DOPA daily for 3 weeks in home cages. For optogenetic testing, L-DOPA was only administered once at the start of the experiment.
ChR2 was activated using a 473-nm DPSS blue laser and NpHR using a 589-nm DPSS yellow laser (Opto Engine LLC) controlled by a Master-9 pulse stimulator (A.M.P.I. Ltd.). For ChR2, the laser was pulsed at 5, 25, 50, and 100 Hz (5 ms square pulse width; 5 mW at fiber tip). For NpHR, the laser was applied continuously for the indicated duration (10 mW at fiber tip). Mice were tested with only one stimulation frequency per day.
Behavioral testing
To assess forelimb akinesia, we performed a modified suspended forelimb stepping test on a treadmill moving at 5 cm/s.37 Forepaw adjusting steps were counted from video recordings from five non-consecutive 30-second cycles that included 10 seconds pre-, during-, and post-laser stimulation periods, followed by a 60-second laser-off period. Results are expressed as % of steps taken by the contralesional paw of total steps taken by both paws.
LID was tested in a 35.5 cm diameter clear cylinder open field. Laser light was delivered starting at 20 minutes after L-DOPA injection for 5 cycles of 30-second laser-on and 60-second laser-off periods. Limb and axial dyskinesias were scored separately by a blinded observer with a previously described rating scale over each 30 second laser-on or laser-off period.38 Following LID testing at 40 minutes post L-DOPA injection, we tested stepping to assess whether ChR2 stimulation altered stepping improvement by L-DOPA. We did not score orofacial AIMs due to the inability to accurately assess them in our videos, especially in the larger open field arena (Video 1) and since changes in orofacial AIMs scores mirror limb AIMs when D1- or D2-dopamine receptors are blocked during LID.39
The same cohort of mice were used to assess akinesia improvement and LID modulation in the L-DOPA naïve and chronic L-DOPA states (Figs. 1–3). These mice were assessed on Day 0 for akinesia without L-DOPA followed by LID in the L-DOPA naïve state, and were tested again on Days 21–24 for LID after daily L-DOPA treatment (Fig. 1A). The same eYFP mice served as controls for ChR2 and NpHR groups by application of blue or yellow laser light, respectively.
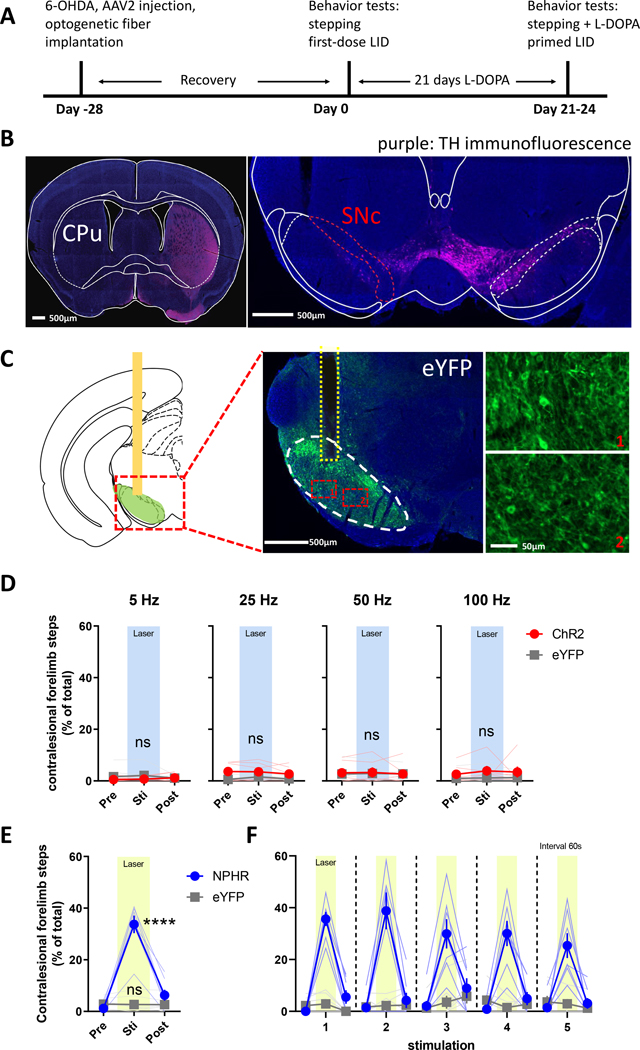
Figure 1. Optogenetic inhibition of SNr GABAergic neurons rescues forelimb akinesia in 6-OHDA-lesioned mice.
(A) Experimental timeline. Mice received unilateral 6-OHDA injections targeting the medial forebrain bundle, followed by AAV2 injection and optic fiber implantation in the ipsilesional SNr. (B) Tyrosine hydroxylase (TH) immunofluorescence (purple) of coronal sections showing the striatum (CPu, left image) and substantia nigra compacta (SNc, right image) from mice with 6-OHDA lesion of the left hemisphere; scale bar = 500 μm. (C) eYFP immunofluorescence (green) of the SNr (white dashed region) and optic fiber location (yellow dashed region) in mice injected with AAV2-eYFP vector; scale bar = 500 μm. Insets show labeling of GABAergic neuron soma in ventral regions of the SNr; scale bar = 50 μm. (D and E) Forelimb akinesia was assessed with the supported treadmill stepping task in mice expressing AAV2-delivered ChR2, NpHR, or eYFP to measure the number of steps taken with the impaired (contralesional) forelimb. (D) Stepping was unaltered by delivery of blue laser light at 5–100 Hz in mice expressing ChR2, but (E) was rescued during application of continuous yellow laser light in mice expressing NpHR (**** P < 0.0001 vs. pre-laser; P < 0.0001 vs. eYFP stim). (F) Akinesia improvement was time-locked to the delivery of yellow laser light and durable over repeated trials. N=6 mice for ChR2, N=7 mice for NpHR and N=7 mice for eYFP.
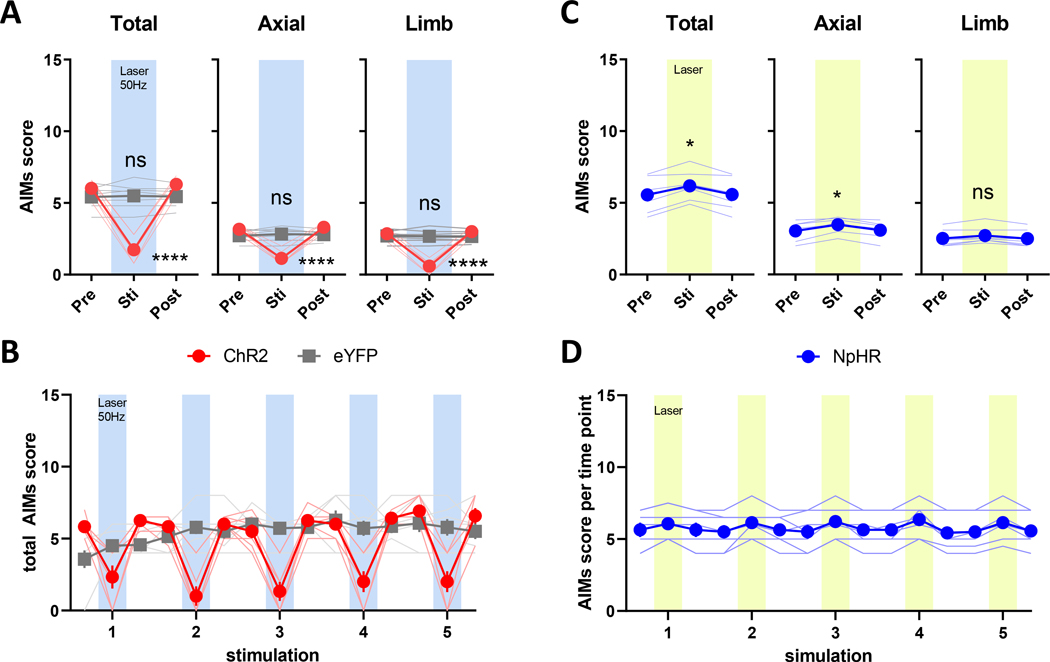
Figure 3. Optogenetic modulation of LID in L-DOPA naïve mice.
Following recovery from 6-OHDA lesion, mice were injected with L-DOPA and scored for AIMs starting at 20 minutes post injection. 30s of blue laser light at 50 Hz or 30s of continuous yellow laser light was applied to stimulate ChR2 and NpHR, respectively. (A) Stimulation of ChR2 decreased total AIMs scores, including axial and limb subscores (B) only during laser-on periods, returning to baseline levels immediately follow laser-offset (**** P < 0.0001 vs. pre-laser, P < 0.0001 vs. eYFP stim). (C) Total AIMs scores and the axial subscore were modestly potentiated by stimulation of NpHR with yellow laser light (D) during laser-on periods (* P < 0.05, Dunn’s posttest following Friedman test, ns: P > 0.05). N=6 mice for ChR2, N=7 mice for NpHR and N=7 mice for eYFP.
Open field mouse tracking and trajectory analysis
Animals were tracked in the open field with overhead videos from the LID tests and were analyzed with DeepLabCut for markerless pose estimation.40 Trajectories were generated from the body center and analyzed using a custom R script (R Project) to assess distance travelled, ipsi- and contralesional rotations, and net cumulative rotation angle. See details in supplemental information. Code available upon request.
Immunohistochemistry
Upon study completion, mice were perfusion fixed with 4% paraformaldehyde and brain tissue processed for fluorescence immunohistochemistry as previously described.34 20 μm free-floating sections were labeled with chicken anti-GFP (1:2000, abcam) and rabbit anti-TH (1:2000, Pel-Freez) antibodies followed by Alexa Fluor-conjugated secondary antibodies (1:500, Thermo Fisher), and scanned with an Olympus VS120 slide scanner.
Statistical analysis
Data are expressed as the mean ± standard error of mean and were analyzed with GraphPad Prism v9.4 (GraphPad Software). The effect of laser stimulation and virus on behavioral outcomes were tested with two-way repeated measures (RM) ANOVA followed by Bonferroni’s multiple comparison posttest. This parametric test allows testing for interaction between two factors for subject-matched data, which is not readily available as a nonparametric test. The effect of one factor RM was tested by the Friedman test (nonparametric), followed by Dunn’s multiple comparison posttest. Additionally, the Friedman and Dunn’s tests were run on each factor of two-way analyses separately, which were generally concordant with the parametric test. All statistical analyses are reported in Supplementary Table S1. P < 0.05 was considered statistically significant.
RESULTS
Optogenetic inhibition of SNr GABAergic neuron activity ameliorates akinesia in 6-OHDA lesioned mice.
Based on the classical rate model of basal ganglia function, striatal DA depletion is predicted to increase the activity of SNr neurons, which inhibits motor output leading to PD-related akinesia.13,14 To determine whether direct modulation of SNr neurons alters akinesia after DA-depletion, we used a well characterized mouse model of parkinsonism induced by unilateral 6-OHDA infusion targeting the MFB,38,41 which results in severe loss of substantia nigra pars compacta (SNc) dopaminergic neurons and their striatal terminals (Fig. 1A–B). Accordingly, akinesia manifests contralateral to the DA lesion, which we assay by counting supported treadmill forepaw adjusting steps (stepping).36 We modulated SNr GABAergic neuron activity by delivering Cre-dependent AAV2 vectors into the SNr ipsilateral to DA lesions in Vgat-Cre mice from which ChR2 or NpHR is expressed to increase or decrease neuronal activity, respectively. Our injections strongly transduced dorsal GABAergic SNr neurons adjacent to the SNc, with sparser labeling of ventral neurons. Optical fibers were implanted over the ipsilateral SNr, allowing us to manipulate neuronal activity at the soma of SNr GABAergic neurons (Fig. 1C and Fig. S1A–C). ChR2 was activated using blue laser light at 5, 25, 50, and 100 Hz, which was previously shown to increase the spiking activity of SNr GABAergic neurons.42,43 Optogenetic stimulation did not affect akinesia of the contralesional forepaw of DA depleted animals or the number of steps taken by the intact ipsilesional forepaw (Fig. 1D, S2A). By contrast, when we inhibited SNr neurons with constant yellow laser light delivered to NpHR-transduced DA-depleted mice, they increased contralesional forelimb use both as a percentage of total steps taken by both limbs (Fig. 1E) and total steps taken (Fig. S2C). As a control, we tested application of blue or yellow laser light in DA-lesioned animals expressing an AAV-eYFP vector, which did not alter forelimb akinesia (Fig. 1D–1F, S2B). The anti-akinetic effect of NpHR was entirely dependent on laser stimulation as akinesia quickly reemerged when the laser was turned off; akinesia improvement was stable across cycles of light stimulation (Fig. 1F).
Regulation of LID by optogenetic upregulation or inhibition of SNr GABAergic neuron activity.
The classical rate model also stipulates that LID results from excessive inhibition of the SNr and GPi due to hyperactivity of striatonigral spiny projection neurons.5,6,44 We next investigated whether direct manipulation of SNr neuron activity could alter LID in our mouse model.38,41 We induced dyskinesia in the same mice expressing ChR2 or NpHR from the akinesia studies by administering L-DOPA (3 mg/kg, IP) daily for 3 weeks, which caused the development of AIMs characteristic of this well-studied model of LID (Fig. 1A).45 Total AIMs scores peaked at 20 minutes post L-DOPA injection and lasted beyond 60 minutes, consistent with the typical 80–120 minute duration of LID in our hands and others using this dose of L-DOPA (Fig. S3C).39,46 We found that increasing SNr neuron activity in ChR2-mice with blue laser light at 25–100 Hz significantly suppressed AIMs scores (Fig. 2A and Video 1). Because stimulation at both 50 Hz and 100 Hz were equally effective (Fig. 2A, 50 vs. 100 Hz; P=0.1135 Bonferroni’s posttest following two-way RM ANOVA) and a study showed that SNr neurons could reliably follow 50 Hz stimulation with spike trains at the same frequency (and not at 100 Hz) with this opsin,42 we used 50 Hz stimulation of ChR2 for the remainder of our studies. Importantly, the reduction of LID was dependent on ChR2, as AIMs scores were unchanged by the same laser stimulation in mice expressing an eYFP control vector, indicating that onset of laser light did not serve as sensory stimulus to interrupt dyskinesia expression (Fig. 2A, 2C, 2D). Pre-stimulation AIMs scores remained stable across the 4 consecutive days of ChR2 stimulation, indicating that LIDs were not permanently altered by daily optostimulation (Fig S3B).
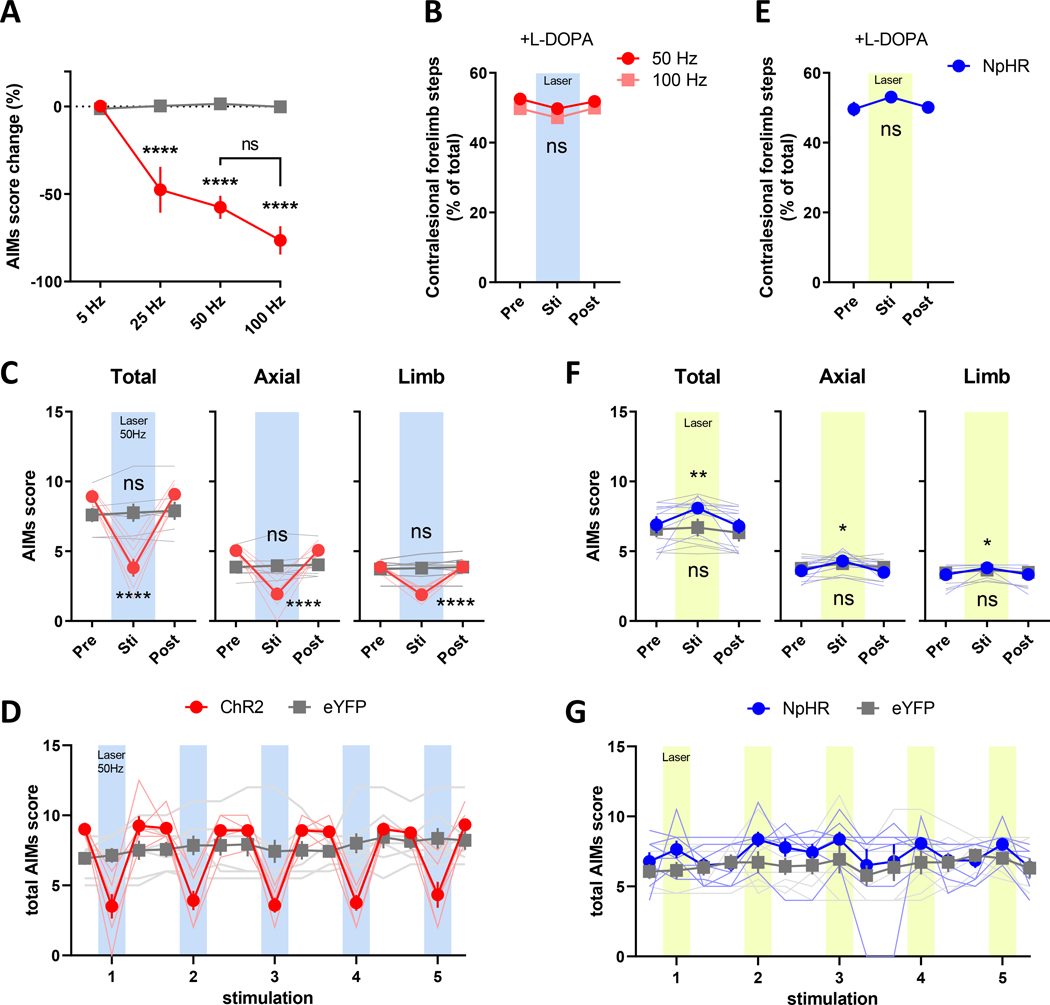
Figure 2. Optogenetic modulation of LID in L-DOPA-primed mice.
6-OHDA-lesioned mice were given L-DOPA once daily (3 mg/kg, IP) for 3 weeks to induce stable dyskinesia. On the day of the LID test, mice were injected with L-DOPA and scored for AIMs starting at 20 minutes post injection. 30s of blue laser light at the indicated frequencies or 30s continuous yellow laser light was applied to stimulate ChR2 and NpHR, respectively. (A) Stimulation of ChR2 decreased total AIMs scores starting at 25 Hz (**** P < 0.0001 vs. laser-off and vs. eYFP at the same frequency), and (B) did not alter the efficacy of L-DOPA in normalizing adjusting forelimb steps. (C) Total AIMs and axial and limb subscores were decreased by ChR2 stimulation (tested at 50 Hz; **** P < 0.0001 vs. pre-laser, P ≤ 0.001 vs. eYFP stim). (D) Reduction in AIMs scores occurred only during laser-on periods and returned to baseline levels immediately following laser-offset. (E) NpHR inhibition of SNr GABAergic neurons did not alter the efficacy of L-DOPA in normalizing forelimb adjusting steps. (F) Total AIMs scores and axial and limb subscores were modestly potentiated by stimulation of NpHR with yellow laser light (G) during laser-on periods (* P < 0.05, ** P < 0.01 vs. pre-laser; P > 0.05 vs. eYFP stim). Supported forelimb adjusting steps were tested at 40 minutes post L-DOPA injection. ns: P > 0.05, N=6 mice for ChR2, N=7 mice for NpHR and N=7 mice for eYFP.
Both axial and limb AIMs scores were similarly reduced (Fig. 2C), and LID improvement was reproducibly time-locked to the period of laser stimulation in ChR2-expressing mice (Fig. 2D). In our PD/LID model, L-DOPA improves akinesia of the impaired contralesional forelimb.47 We next asked whether increasing SNr GABAergic neuron activity interfered with this therapeutic effect by evaluating the improvement in forepaw stepping after L-DOPA administration at a time point after peak LID (40 minutes) since AIMs interfere with performance of the stepping task. Contralesional forepaw use was nearly normalized by L-DOPA, which is unaltered by stimulation of ChR2 at frequencies that improved LID (Fig. 2B, S3D), indicating that our stimulation protocol improves LID while preserving the anti-akinetic effects of L-DOPA. This was also evident in the open field as mice transitioned from AIMs to more linear locomotion upon the onset of ChR2 laser stimulation even during peak LID, signified by decreased contralesional turning while maintaining the same distance travelled as evident in their trajectories (Video 1, Fig. S4A–C, S4K–M).
Based on these findings, we predicted that inhibition of SNr neurons with NpHR would worsen LID. As with the ChR2 studies, we induced LID in NpHR-transduced mice and found that yellow laser stimulation modestly increased AIMs scores, similarly increasing both limb and axial AIMs (Fig. 2F). This effect was confined to the duration of laser light application (Fig. 2G) and did not alter L-DOPA-mediated improvement in contralesional forelimb stepping (Fig. 2E, S3E).
In mouse models of severe DA depletion, LID is induced even by the first dose of L-DOPA and increases by priming with repeated dosing (Fig. S3B).45 We found that modulating SNr neuron activity with ChR2 decreased LID and NpHR potentiated LID induced by the first L-DOPA dose (Fig. 3). The magnitude of this effect was similar to that in primed animals, though overall AIMs scores were lower as is expected for unprimed animals (Fig. 2, 3, S3B).
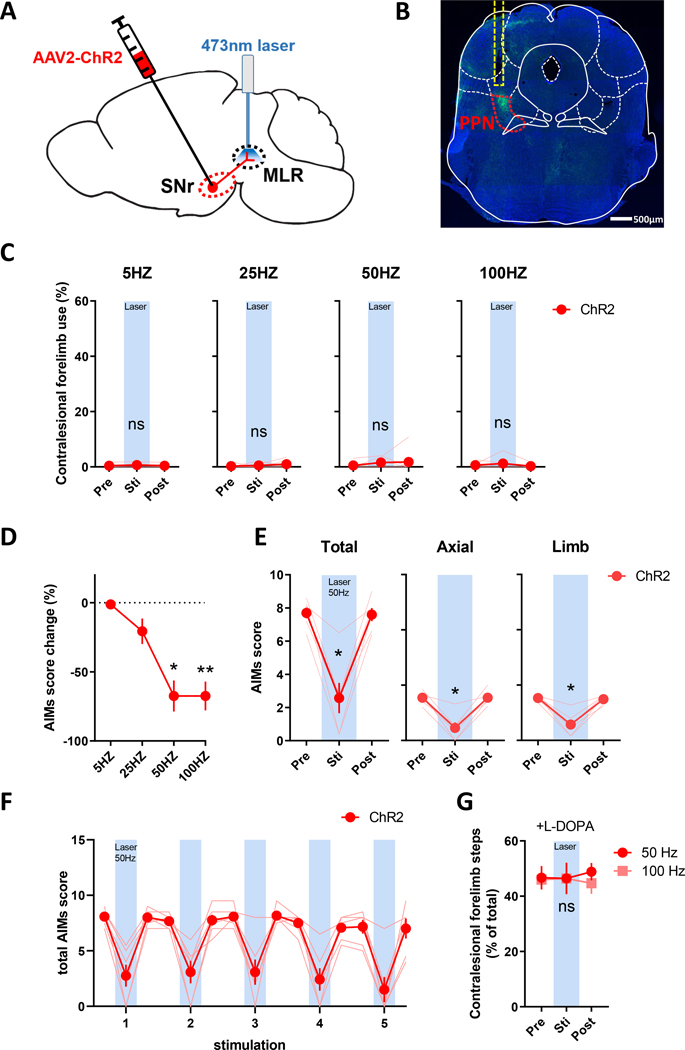
Optogenetic stimulation ChR2 of SNr terminals in the PPN terminals suppresses LID.
As the primary rodent basal ganglia output nucleus, the SNr is interconnected with numerous nuclei including the PPN, a midbrain region critical for locomotion.48,49 We and others have shown that markers of neuronal activity are increased in the PPN following L-DOPA administration in rodent LID models.34,50 As such, we tested whether suppression of LID by ChR2 activation in the SNr was mediated through their projections to PPN. A new cohort of 6-OHDA lesioned mice received infusions of AAV-ChR2 in the SNr, while an optical fiber was implanted above the ipsilateral PPN (Fig. 4A). Expression of ChR2 in SNr projection fibers and proper optical fiber placement were verified by immunofluorescence (Fig. 4B and Fig. S1D). We tested blue laser light stimulation at 5, 25, 50, and 100 Hz applied to SNr-PPN fibers and found that 50 and 100 Hz stimulation reduced AIMs scores in L-DOPA primed mice (Fig. 4D and Video 2) similar to SNr stimulation. Likewise, AIMs were only suppressed during laser stimulation with both limb and axial AIMs scores similarly reduced (Fig. 4E–F). Stimulation of SNr-PPN fibers alone neither altered contralesional forepaw akinesia (Fig. 4C) nor interfered with L-DOPA-mediated akinesia improvement (Fig. 4G, S3D), and was effective in reducing LID with the first L-DOPA dose (total AIMs: 6.3 ± 0.44 laser-off, 3.6 ± 0.83 laser-on; p<0.01). Together, the similarity between ChR2 stimulation at SNr neuronal soma and SNr-to-PPN fibers support that LID improvement from increasing SNr neuronal activity is mediated though its projections to the PPN.
Figure 4. Optogenetic stimulation of ChR2 in SNr to PPN fibers attenuates LID.
Mice received unilateral 6-OHDA lesion and (A) injection of AAV2-ChR2 in the SNr and optic fiber implantation in the PPN ipsilateral to the DA lesion. (B) eYFP immunofluorescence (green) of SNr fibers in the PPN (red dashed region) and optic fiber location (yellow dashed region); scale bar = 500 μm. (C) Stimulation of ChR2 from SNr fibers in the PPN with blue laser light does not alter forelimb akinesia, but (D) decreased total AIMs scores at 50 and 100 Hz (* P < 0.05, ** P < 0.01 vs. pre-laser, Dunn’s posttest following Friedman test). (E-F) Both axial and limb subscores and total AIMS were reduced reproducibly during 50 Hz blue laser-on periods (* P < 0.05 vs. pre-laser, Dunn’s posttest following Friedman test). (G) Stimulation of SNr fibers in the PPN did not alter L-DOPA-mediated improvement in forelimb adjusting steps measured at 40 minutes post L-DOPA injection (ns: P > 0.05 vs. pre-laser).
DISCUSSION
To our knowledge, this is the first study to selectively modulate SNr GABAergic neurons with optogenetics in a rodent PD/LID model. Our data indicate that decreasing SNr GABAergic neuron activity ameliorates PD symptoms, which supports the classical model stating that basal ganglia output hyperactivity is associated with PD akinesia.21,22 We also show that increasing SNr GABAergic neuron activity suppresses LID while preserving the effect of L-DOPA in improving supported contralesional forepaw stepping, supporting the classic understanding of LID pathophysiology where dopamine triggers excessive SNr/GPi inhibition.21,22 Notably, mice transition from AIMs to nearly normal locomotion upon optogenetic stimulation of ChR2 (Video 1, Fig. S4), which suggests that our stimulation paradigm does not suppress motor activity in total as is the case with some other antidyskinetic approaches.33,51,52 Importantly, we reproduced this by stimulating SNr projection fibers in the PPN, identifying a potential SNr target that mediates LID. As these SNr projections are GABAergic, increasing SNr activity likely decreases the activity of target neurons, supporting that hyperactivity of PPN neurons may arise from DA-depletion and chronic L-DOPA treatment.
Though DBS of the STN and GPi are effective and widely used interventions to improve motor symptoms and reduce dyskinesia in PD, the precise mechanisms by which DBS is effective remain unsettled, especially whether the therapeutic actions result from increasing or decreasing the activity of targeted neurons and/or passing fibers.53 Further, improvement of LID following surgical STN or GPi lesions, which should eliminate output from these structures, is inconsistent with the classical rate model prediction that LIDs are caused by excessive suppression of SNr/GPi activity.23 Alternatively, DBS may disrupt aberrant firing patterns that pervade the cortical-basal ganglia-thalamic motor loop in PD, including in the SNr.54–57 Indeed, altered firing patterns following DA-depletion (increased burstiness, oscillatory activity, and synchrony) are consistently reported in the SNr, despite disparate findings regarding changes in firing rate.24,25 Our data show that direct modulation of SNr neurons in a manner to counteract the predicted changes in firing rate in PD and LID improves akinesia and reduces LID as predicted by the classic rate model, and suggests that the threshold for LID improvement by increasing SNr firing rate is lower than that needed to cause akinesia. Whether DBS ultimately works to improve LID by increasing SNr/GPi output to select targets such as the PPN as reported in nonhuman primates58 or whether our optogenetic manipulations also normalize aberrant firing patterns remains to be elucidated. Our manipulations of SNr activity equally decreased LID with the first dose and in primed animals, suggesting they reduce the expression of LID independent of the mechanisms that sensitize response to DA, perhaps downstream of changes to basal ganglia plasticity that occur from priming by chronic L-DOPA treatment in the DA-depleted state.59,60 That both axial and limb AIMs scores were similarly reduced is consistent with our manipulations being downstream of the striatum where preferential modulation of D1- or D2-receptor mediated pathways can show differential effect on AIMs subtypes, including orofacial dyskinesias that were not scored in our study.39,47
In this study, we considered the mouse SNr to be functionally analogous to the GPi in humans,61 though the mouse entopeduncular nucleus (EP) is the anatomical GPi analog.62 Neuromodulation of the EP can also improve parkinsonism and LID in rodent models.26,63–65 These two structures serve as the output nuclei of the basal ganglia, and though are often considered one unit, are likely involved in unique processes.9,66 Though we specifically targeted SNr GABAergic neurons, understanding whether the regulation of LID is segregated among these nuclei and how this maps to humans will be necessary to move forward for clinical application.
Our finding that optogenetic stimulation SNr fibers in the PPN decreases LID indicates that this region may facilitate hyperkinesia following L-DOPA administration. Retrograde tracing studies show that the majority of SNr fibers in this midbrain region target the PPN (versus the CnF), suggesting that the PPN likely mediates the effect of SNr cell body and fiber stimulation in our study.67 Containing glutamatergic, GABAergic, and cholinergic neurons, the PPN integrates diverse inputs68 and projects to numerous ascending and descending target regions.69 PPN dysfunction has received significant attention for PD-associated akinesia30–32 and LID50 in animal models, and has been proposed as a target for DBS.70,71 Indeed, chemogenetic upregulation of PPN cholinergic neurons in a lactacystin partial lesion PD model improved measures of akinesia,30,31 while both chemogenetic and optogenetic activation of caudal PPN glutamatergic neurons rescued motor deficits caused by acute loss of dopamine signaling from D1- and D2-dopamine receptor antagonists.32 This suggests that parkinsonian akinesia may result from abnormal activity of these PPN neurons, which may arise from increased GABAergic output from the SNr,32 consistent with our observed improvement of akinesia by SNr inhibition (Fig. 1E). We will address this in future studies by inhibiting SNr terminals in the PPN with other inhibitory opsins that overcome limitations of NpHR for presynaptic terminal silencing.72
That PPN neurons are hyperactive during LID is supported by increased expression of activity markers following L-DOPA administration in primed rodents as we and other previously reported,33,34 and increased firing rates of PPN neurons recorded in vivo following 6-OHDA lesions in rodents.73 Selective optogenetic activation of PPN neuron types in dopamine-intact animals has yielded varying results, though short duration stimulation of GABAergic PPN neurons consistently slowed ongoing locomotion.74,75 However, the activation of PPN cholinergic neurons has been reported to increase spontaneous movement,76 or increase75 or decrease74 speed only of ongoing locomotion. Likewise, stimulation of glutamatergic PPN neurons can initiate locomotion or increase the speed of ongoing locomotion,32,74,75,77 or inhibit movement,67,77 highlighting the functional heterogeneity of this population of neurons defined by anatomical location within the PPN and downstream targets (i.e., to the spinal cord, brainstem, basal ganglia).67,74,77 It is tempting to speculate that the population of locomotion-activating glutamatergic PPN neurons become hyperactive during LID and are suppressed by increasing GABA release from SNr terminals in our study; however, determining which neuron type(s) are dysregulated during LID will be paramount for understanding how SNr modulation of PPN neurons decreases LID. It is worth noting that targeted ablation of cholinergic PPN neurons yielded only modest changes in LID,78 supporting a role for the glutamatergic or GABAergic population. While we showed that stimulation of SNr to PPN fibers was sufficient to reduce LID, we have not excluded contributions of other SNr targets.
In summary, we show that optogenetic inhibition of SNr activity improved akinesia while optogenetic stimulation reduced LID and allowed normal locomotion. Stimulation of SNr fibers in the PPN recapitulated the antidyskinetic effect of somatic stimulation, identifying this region as a potential target for LID management. These results will help us to further understand the circuit dysregulation that underlies LID and may provide new approaches for PD treatment.
Supplementary Material
Acknowledgments:
This research was supported by NIH Grant R21NS108068 (UJK), and Parkinson’s Foundation (YH, SLA).
Footnotes
Financial Disclosure/Conflict of Interest statement: The authors declare no conflict of interest.
References
- 1.Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med 2003;348(14):1356–1364. [DOI] [PubMed] [Google Scholar]
- 2.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron 2003;39(6):889–909. [DOI] [PubMed] [Google Scholar]
- 3.Hornykiewicz O Neurochemistry of parkinsonism. Handbook of Neurochemistry 1972;7:465–501. [Google Scholar]
- 4.Jellinger K The pathology of parkinsonism. In: Marsden CD, Fahn S, eds. Movement Disorders 2. London: Butterworths, 1987:124–165. [Google Scholar]
- 5.Jenner P Molecular mechanisms of L-DOPA-induced dyskinesia. Nature reviews Neuroscience 2008;9(9):665–677. [DOI] [PubMed] [Google Scholar]
- 6.Bastide MF, Meissner WG, Picconi B, et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 2015;132:96–168. [DOI] [PubMed] [Google Scholar]
- 7.Picconi B, Centonze D, Hakansson K, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nature neuroscience 2003;6(5):501–506. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang X, Mazzoni P, Kang UJ. The role of neuroplasticity in dopaminergic therapy for Parkinson disease. Nat Rev Neurol 2013;9(5):248–256. [DOI] [PubMed] [Google Scholar]
- 9.Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, et al. Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Disord 2008;23 Suppl 3:S548–559. [DOI] [PubMed] [Google Scholar]
- 10.Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci 2010;11(11):760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med 2010;362(22):2077–2091. [DOI] [PubMed] [Google Scholar]
- 12.Chiken S, Nambu A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist 2016;22(3):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in neurosciences 1989;12(10):366–375. [DOI] [PubMed] [Google Scholar]
- 14.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990;13(7):281–285. [DOI] [PubMed] [Google Scholar]
- 15.Burbaud P, Gross C, Benazzouz A, Coussemacq M, Bioulac B. Reduction of apomorphine-induced rotational behaviour by subthalamic lesion in 6-OHDA lesioned rats is associated with a normalization of firing rate and discharge pattern of pars reticulata neurons. Exp Brain Res 1995;105(1):48–58. [DOI] [PubMed] [Google Scholar]
- 16.Breit S, Martin A, Lessmann L, Cerkez D, Gasser T, Schulz JB. Bilateral changes in neuronal activity of the basal ganglia in the unilateral 6-hydroxydopamine rat model. J Neurosci Res 2008;86(6):1388–1396. [DOI] [PubMed] [Google Scholar]
- 17.Willard AM, Isett BR, Whalen TC, et al. State transitions in the substantia nigra reticulata predict the onset of motor deficits in models of progressive dopamine depletion in mice. eLife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaville C, Navailles S, Benazzouz A. Effects of noradrenaline and serotonin depletions on the neuronal activity of globus pallidus and substantia nigra pars reticulata in experimental parkinsonism. Neuroscience 2012;202:424–433. [DOI] [PubMed] [Google Scholar]
- 19.Tseng KY, Kargieman L, Gacio S, Riquelme LA, Murer MG. Consequences of partial and severe dopaminergic lesion on basal ganglia oscillatory activity and akinesia. Eur J Neurosci 2005;22(10):2579–2586. [DOI] [PubMed] [Google Scholar]
- 20.MacLeod NK, Ryman A, Arbuthnott GW. Electrophysiological properties of nigrothalamic neurons after 6-hydroxydopamine lesions in the rat. Neuroscience 1990;38(2):447–456. [DOI] [PubMed] [Google Scholar]
- 21.Galvan A, Devergnas A, Wichmann T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front Neuroanat 2015;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obeso JA, Rodriguez-Oroz MC, Rodriguez M, et al. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci 2000;23(10 Suppl):S8–19. [DOI] [PubMed] [Google Scholar]
- 23.Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease. Brain 1994;117 ( Pt 4):877–897. [DOI] [PubMed] [Google Scholar]
- 24.Aristieta A, Ruiz-Ortega JA, Miguelez C, Morera-Herreras T, Ugedo L. Chronic L-DOPA administration increases the firing rate but does not reverse enhanced slow frequency oscillatory activity and synchronization in substantia nigra pars reticulata neurons from 6-hydroxydopamine-lesioned rats. Neurobiol Dis 2016;89:88–100. [DOI] [PubMed] [Google Scholar]
- 25.Meissner W, Ravenscroft P, Reese R, et al. Increased slow oscillatory activity in substantia nigra pars reticulata triggers abnormal involuntary movements in the 6-OHDA-lesioned rat in the presence of excessive extracellular striatal dopamine. Neurobiol Dis 2006;22(3):586–598. [DOI] [PubMed] [Google Scholar]
- 26.Alam M, Capelle HH, Schwabe K, Krauss JK. Effect of deep brain stimulation on levodopa-induced dyskinesias and striatal oscillatory local field potentials in a rat model of Parkinson’s disease. Brain Stimul 2014;7(1):13–20. [DOI] [PubMed] [Google Scholar]
- 27.Gago B, Marin C, Rodriguez-Oroz MC, Obeso JA. l-dopa-induced dyskinesias in unilateral 6-hydroxydopamine-lesioned rats are not modified by excitotoxic lesion of the entopeduncular nucleus and substantia nigra pars reticulata. Synapse 2013;67(7):407–414. [DOI] [PubMed] [Google Scholar]
- 28.Zhou FM. Chapter 15 - The Substantia Nigra Pars Reticulata. In: Steiner H, Tseng KY, eds. Handbook of Behavioral Neuroscience: Elsevier, 2016:293–316. [Google Scholar]
- 29.Antal M, Beneduce BM, Regehr WG. The substantia nigra conveys target-dependent excitatory and inhibitory outputs from the basal ganglia to the thalamus. The Journal of neuroscience : the official journal of the Society for Neuroscience 2014;34(23):8032–8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pienaar IS, Gartside SE, Sharma P, et al. Pharmacogenetic stimulation of cholinergic pedunculopontine neurons reverses motor deficits in a rat model of Parkinson’s disease. Mol Neurodegener 2015;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma PK, Wells L, Rizzo G, et al. DREADD Activation of Pedunculopontine Cholinergic Neurons Reverses Motor Deficits and Restores Striatal Dopamine Signaling in Parkinsonian Rats. Neurotherapeutics 2020;17(3):1120–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masini D, Kiehn O. Targeted activation of midbrain neurons restores locomotor function in mouse models of parkinsonism. Nature communications 2022;13(1):504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bastide MF, Dovero S, Charron G, et al. Immediate-early gene expression in structures outside the basal ganglia is associated to l-DOPA-induced dyskinesia. Neurobiol Dis 2014;62:179–192. [DOI] [PubMed] [Google Scholar]
- 34.Ding Y, Restrepo J, Won L, Hwang DY, Kim KS, Kang UJ. Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson’s disease. Neurobiol Dis 2007;27(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vong L, Ye C, Yang Z, Choi B, Chua S Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011;71(1):142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Won L, Ding Y, Singh P, Kang UJ. Striatal cholinergic cell ablation attenuates L-DOPA induced dyskinesia in Parkinsonian mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 2014;34(8):3090–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neuroscience 1999;88(2):617–628. [DOI] [PubMed] [Google Scholar]
- 38.Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis 2004;16(1):110–123. [DOI] [PubMed] [Google Scholar]
- 39.Andreoli L, Abbaszadeh M, Cao X, Cenci MA. Distinct patterns of dyskinetic and dystonic features following D1 or D2 receptor stimulation in a mouse model of parkinsonism. Neurobiol Dis 2021:105429. [DOI] [PubMed] [Google Scholar]
- 40.Mathis A, Mamidanna P, Cury KM, et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nature neuroscience 2018;21(9):1281–1289. [DOI] [PubMed] [Google Scholar]
- 41.Francardo V, Recchia A, Popovic N, Andersson D, Nissbrandt H, Cenci MA. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiol Dis 2011;42(3):327–340. [DOI] [PubMed] [Google Scholar]
- 42.Toda K, Lusk NA, Watson GDR, et al. Nigrotectal Stimulation Stops Interval Timing in Mice. Curr Biol 2017;27(24):3763–3770 e3763. [DOI] [PubMed] [Google Scholar]
- 43.Hormigo S, Vega-Flores G, Castro-Alamancos MA. Basal Ganglia Output Controls Active Avoidance Behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 2016;36(40):10274–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papa SM, Desimone R, Fiorani M, Oldfield EH. Internal globus pallidus discharge is nearly suppressed during levodopa-induced dyskinesias. Annals of neurology 1999;46(5):732–738. [DOI] [PubMed] [Google Scholar]
- 45.Ding Y, Won L, Britt JP, Lim SA, McGehee DS, Kang UJ. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proceedings of the National Academy of Sciences of the United States of America 2011;108(2):840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi SJ, Ma TC, Ding Y, et al. Alterations in the intrinsic properties of striatal cholinergic interneurons after dopamine lesion and chronic L-DOPA. eLife 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alcacer C, Andreoli L, Sebastianutto I, Jakobsson J, Fieblinger T, Cenci MA. Chemogenetic stimulation of striatal projection neurons modulates responses to Parkinson’s disease therapy. J Clin Invest 2017;127(2):720–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira-Pinto MJ, Kanodia H, Falasconi A, Sigrist M, Esposito MS, Arber S. Functional diversity for body actions in the mesencephalic locomotor region. Cell 2021;184(17):4564–4578 e4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arber S, Costa RM. Networking brainstem and basal ganglia circuits for movement. Nat Rev Neurosci 2022;23(6):342–360. [DOI] [PubMed] [Google Scholar]
- 50.Chambers NE, Lanza K, Bishop C. Pedunculopontine Nucleus Degeneration Contributes to Both Motor and Non-Motor Symptoms of Parkinson’s Disease. Front Pharmacol 2019;10:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bezard E, Munoz A, Tronci E, et al. Anti-dyskinetic effect of anpirtoline in animal models of L-DOPA-induced dyskinesia. Neurosci Res 2013;77(4):242–246. [DOI] [PubMed] [Google Scholar]
- 52.Iravani MM, Tayarani-Binazir K, Chu WB, Jackson MJ, Jenner P. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates, the selective 5-hydroxytryptamine 1a agonist (R)-(+)-8-OHDPAT inhibits levodopa-induced dyskinesia but only with\ increased motor disability. J Pharmacol Exp Ther 2006;319(3):1225–1234. [DOI] [PubMed] [Google Scholar]
- 53.Herrington TM, Cheng JJ, Eskandar EN. Mechanisms of deep brain stimulation. J Neurophysiol 2016;115(1):19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kopell BH, Rezai AR, Chang JW, Vitek JL. Anatomy and physiology of the basal ganglia: implications for deep brain stimulation for Parkinson’s disease. Mov Disord 2006;21 Suppl 14:S238–246. [DOI] [PubMed] [Google Scholar]
- 55.Eisinger RS, Cernera S, Gittis A, Gunduz A, Okun MS. A review of basal ganglia circuits and physiology: Application to deep brain stimulation. Parkinsonism Relat Disord 2019;59:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wichmann T, DeLong MR. Deep Brain Stimulation for Movement Disorders of Basal Ganglia Origin: Restoring Function or Functionality? Neurotherapeutics 2016;13(2):264–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson LA, Wang J, Nebeck SD, Zhang J, Johnson MD, Vitek JL. Direct Activation of Primary Motor Cortex during Subthalamic But Not Pallidal Deep Brain Stimulation. J Neurosci 2020;40(10):2166–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Wang ZI, Baker KB, Vitek JL. Effect of globus pallidus internus stimulation on neuronal activity in the pedunculopontine tegmental nucleus in the primate model of Parkinson’s disease. Exp Neurol 2012;233(1):575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bove F, Calabresi P. Chapter 11 - Plasticity, genetics, and epigenetics in l-dopa-induced dyskinesias. In: Quartarone A, Ghilardi MF, Boller F, eds. Handbook of Clinical Neurology: Elsevier, 2022:167–184. [DOI] [PubMed] [Google Scholar]
- 60.Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients. Brain 2009;132(Pt 2):309–318. [DOI] [PubMed] [Google Scholar]
- 61.Deniau JM, Mailly P, Maurice N, Charpier S. The pars reticulata of the substantia nigra: a window to basal ganglia output. Progress in brain research 2007;160:151–172. [DOI] [PubMed] [Google Scholar]
- 62.Pollack AE. Anatomy, physiology, and pharmacology of the basal ganglia. Neurol Clin 2001;19(3):523–534, v. [DOI] [PubMed] [Google Scholar]
- 63.Assaf F, Schiller Y. A chemogenetic approach for treating experimental Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 2019;34(4):469–479. [DOI] [PubMed] [Google Scholar]
- 64.Yoon HH, Nam MH, Choi I, Min J, Jeon SR. Optogenetic inactivation of the entopeduncular nucleus improves forelimb akinesia in a Parkinson’s disease model. Behav Brain Res 2020;386:112551. [DOI] [PubMed] [Google Scholar]
- 65.Moon HC, Won SY, Kim EG, Kim HK, Cho CB, Park YS. Effect of optogenetic modulation on entopeduncular input affects thalamic discharge and behavior in an AAV2-alpha-synuclein-induced hemiparkinson rat model. Neurosci Lett 2018;662:129–135. [DOI] [PubMed] [Google Scholar]
- 66.Nelson AB, Kreitzer AC. Reassessing models of basal ganglia function and dysfunction. Annu Rev Neurosci 2014;37:117–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dautan D, Kovacs A, Bayasgalan T, Diaz-Acevedo MA, Pal B, Mena-Segovia J. Modulation of motor behavior by the mesencephalic locomotor region. Cell reports 2021;36(8):109594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McElvain LE, Chen Y, Moore JD, et al. Specific populations of basal ganglia output neurons target distinct brain stem areas while collateralizing throughout the diencephalon. Neuron 2021;109(10):1721–1738 e1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mena-Segovia J, Bolam JP. Rethinking the Pedunculopontine Nucleus: From Cellular Organization to Function. Neuron 2017;94(1):7–18. [DOI] [PubMed] [Google Scholar]
- 70.Welter ML, Demain A, Ewenczyk C, et al. PPNa-DBS for gait and balance disorders in Parkinson’s disease: a double-blind, randomised study. J Neurol 2015;262(6):1515–1525. [DOI] [PubMed] [Google Scholar]
- 71.Bourilhon J, Mullie Y, Olivier C, et al. Stimulation of the pedunculopontine and cuneiform nuclei for freezing of gait and falls in Parkinson disease: Cross-over single-blinded study and long-term follow-up. Parkinsonism & related disorders 2022;96:13–17. [DOI] [PubMed] [Google Scholar]
- 72.Mahn M, Prigge M, Ron S, Levy R, Yizhar O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nature neuroscience 2016;19(4):554–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, Geng X, Li M, et al. Electrophysiological and Neurochemical Considerations of Distinct Neuronal Populations in the Rat Pedunculopontine Nucleus and Their Responsiveness Following 6-Hydroxydopamine Lesions. Frontiers in neuroscience 2019;13:1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caggiano V, Leiras R, Goni-Erro H, et al. Midbrain circuits that set locomotor speed and gait selection. Nature 2018;553(7689):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, Kreitzer AC. Cell-Type-Specific Control of Brainstem Locomotor Circuits by Basal Ganglia. Cell 2016;164(3):526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao C, Cho JR, Zhou C, et al. Cholinergic Mesopontine Signals Govern Locomotion and Reward through Dissociable Midbrain Pathways. Neuron 2016;90(2):333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferreira-Pinto MJ, Kanodia H, Falasconi A, Sigrist M, Esposito MS, Arber S. Functional diversity for body actions in the mesencephalic locomotor region. Cell 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chambers NE, Coyle M, Sergio J, et al. Effects of pedunculopontine nucleus cholinergic lesion on gait and dyskinesia in hemiparkinsonian rats. Eur J Neurosci 2021;53(8):2835–2847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.