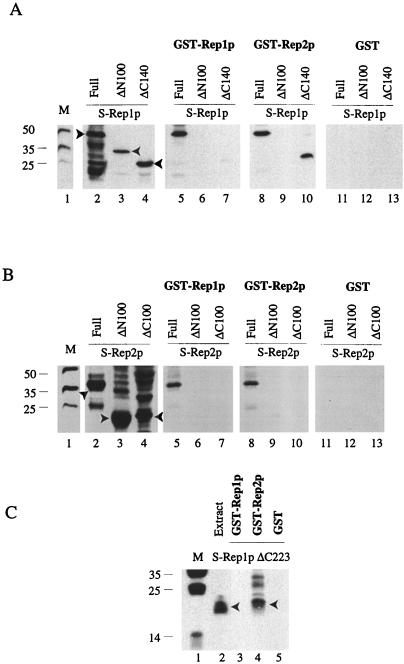
FIG. 4.
Baiting assays for Rep1p and Rep2p and their deletions performed with GST-Rep1p and GST-Rep2p hybrid proteins. The assays were carried out as described by Ahn et al. (1). After baiting, proteins were fractionated in SDS–12% polyacrylamide gels and probed with the S-protein probe. The lane marked M displays the molecular-weight standards. (A and B) In lanes 2 to 4, proteins extracted by boiling the cells from 200-μl portions of the induced cultures (that were also the starting materials for the assays depicted in lanes 5 to 13) in the SDS sample buffer were run as controls. The S-Rep protein bands of interest in lanes 2 to 4 are indicated by the arrowheads. The data in panel A were obtained with S-tagged Rep1p or its derivatives; the data in panel B were obtained with S-tagged Rep2p or its derivatives. The GST-Rep hybrids used as the baits and the corresponding baited S-Rep proteins (either full length or partially deleted) are indicated above lanes 5 to 10. In the reactions represented in lanes 11 to 13, the baiting was performed with GST and not with the GST-Rep hybrids. (C) Results obtained with Rep1pΔC223 fused to the S-peptide tag. Lane 2 represents the extract from 200 μl of the induced culture expressing Rep1pΔC223; lanes 3 to 5 correspond to baiting assays with GST-Rep1p, GST-Rep2p, and GST, respectively. Detailed protocols of the assays are described in Ahn et al. (1).