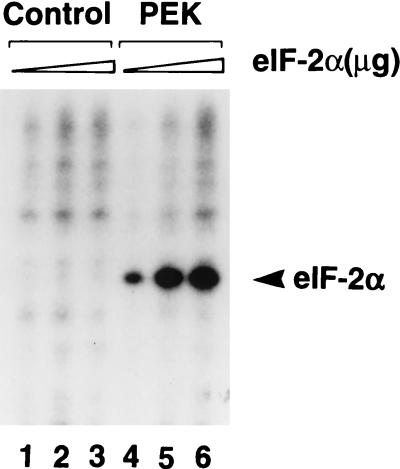
FIG. 5.
Immunoprecipitated PEK phosphorylates human eIF-2α. PEK or an unrelated bacterial protein was expressed in Sf-9 cells and the PEK was immunoprecipitated with polyclonal antisera prepared against a synthetic peptide derived from the carboxy terminus of the kinase. Immunocomplexes prepared from lysates expressing an unrelated bacterial protein (lanes 1 to 3) or PEK (lanes 4 to 6) were incubated with [γ-32P]ATP and increasing amounts of human eIF-2α as described in Materials and Methods. Radiolabeled proteins were separated by electrophoresis with an SDS-polyacrylamide gel and visualized by autoradiography. Kinase assay mixtures contained 1 (lane 1 and 4), 2 (lanes 2 and 5), or 4 (lanes 3 and 6) μg of purified human eIF-2α protein. The arrowhead indicates the position of the phosphorylated eIF-2α.