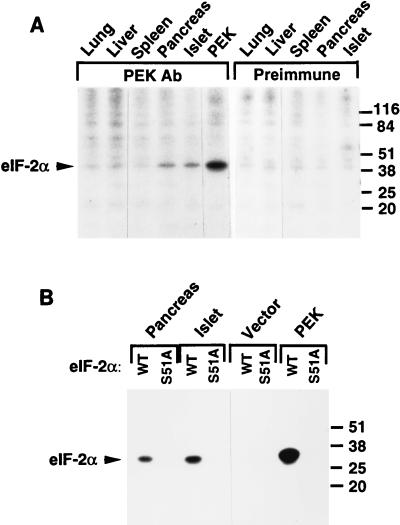
FIG. 8.
PEK immunoprecipitated from mammalian tissues phosphorylates eIF-2α at serine-51. (A) PEK was immunoprecipitated from the indicated tissues by using polyclonal PEK antibody and incubated with [γ-32P]ATP and human eIF-2α as described in Materials and Methods. As a control, a similar immunoprecipitation assay was carried out with preimmune serum. Radiolabeled proteins were separated by SDS-PAGE and were visualized by autoradiography. The arrowhead indicates the position of phosphorylated eIF-2α. All tissues used in this study are from rats, with the exception of islets, which were isolated from dogs. (B) PEK was immunoprecipitated from pancreas, islets, and Sf-9 cells. As a control, similar immunoprecipitations were carried out with lysates prepared from Sf-9 insect cells containing only the vector. Immunoprecipitates were added to kinase reaction mixtures containing [γ-32P]ATP and a modified form of yeast eIF-2 containing wild-type serine-51 (WT) or alanine substituted for serine at this phosphorylation site (S51A). Radiolabeled proteins were analyzed by SDS-PAGE, followed by autoradiography. Sizes of protein standards in kilodaltons on the right sides of both panels.