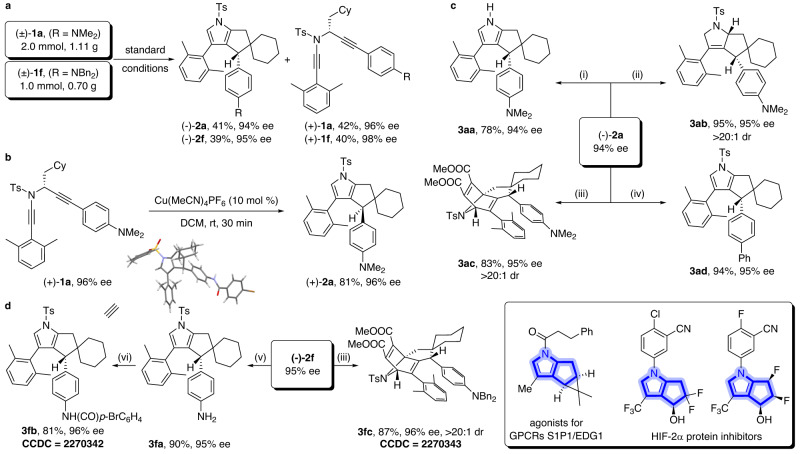
Fig. 4. Scale-up reaction and product elaborations.
a Preparative-scale synthesis of products (−)-2a and (−)-2f. b Synthesis of the chiral product (+)-2a from chiral diyne (+)-1a. c Conversion of product (−)-2a into compounds 3aa–3ad. d Conversion of product (−)-2f into compounds 3fa–3fc. Reagents and conditions: (i) KOH (5 equiv), THF/MeOH = 1:1, 80 °C, 8 h. (ii) NaBH3CN (5 equiv), DCM/TFA = 10:1, 0 °C, 1 h. (iii) dimethyl acetylenedicarboxylate (10 equiv), toluene, 100 °C, 12 h. (iv) PhMgBr (1.2 equiv), Pd(PPh3)2Cl2 (5 mol %), THF, rt, 30 min. (v) Pd/C (10% w/w), H2 (1 atm), EtOH/EtOAc = 1:1, 80 °C, 5 h. (vi) p-BrC6H4COCl (1.5 equiv), Et3N (2 equiv), DCM, rt, 2 h.