Figure 2.
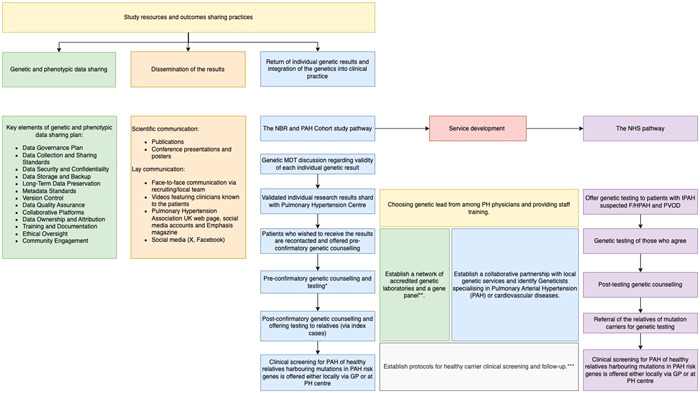
Study resources and outcomes‐sharing practices can be categorized into three main groups. (1) Data sharing plan. Genetic and phenotypic data management and sharing structure were developed at the study's inception and have since undergone continuous refinement to adapt to the evolving requirements and dynamics of the research environment. (2) Dissemination of the results. The findings of the genetic study have been shared through traditional scientific communication channels, including publications, conference presentations, and posters. To reach the general public, we employed face‐to‐face communication facilitated by recruiting/local teams, which patients also preferred. Additionally, dissemination occurred through videos featuring clinicians familiar to the patients, the Pulmonary Hypertension Association webpage, social media accounts, Emphasis magazine, and social media platforms. (3) Return of genetic results. The return of individual genetic results occurred in multiple stages, requiring the development of various local and national services and care pathways. This process resulted in the integration of genetics into clinical care in Pulmonary Arterial Hypertension. *In certain centers, Pulmonary Hypertension Physicians (Respiratory Physicians) directly requested genetic testing, with genetic counselling provided only after the results were available. In contrast, at other centers, patients received preconfirmatory genetic counselling and were notified of the results through a letter. Postconfirmatory counselling was offered only to those patients who expressed an interest in having their relatives tested **Since 2021, national genomic testing service in the United Kingdom has been delivered through a network of seven Genomic Laboratory Hubs (GLHs), each responsible for coordinating services for a particular part of the country. Two GLHs were commissioned to perform “R188 Pulmonary Arterial Hypertension” testing for the National Health Service Genomic Medicine Service, The North East and Yorkshire GLH (Sheff7ield Children's FT) and the South East GLH (Royal Brompton and Harefield FT). Testing criteria include IPAH, suspected F/HPAH, and PVOD. R186 Hereditary hemorrhagic telangiectasia panel is used in patients with PAH and HHT. ***In most PH centers, healthy carrier screening with echocardiogram and cardiopulmonary exercise testing is offered yearly, whereas healthy relatives of patients with the familial form of the disease but without mutations in known PAH risk genes are seen every 2 years (Supporting Information).
