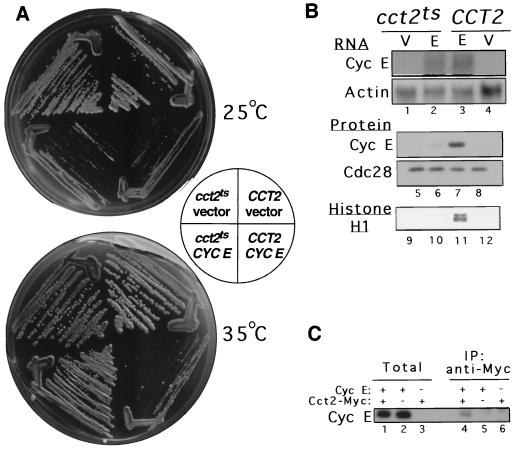
FIG. 1.
A growth-defective phenotype of yeast cells overexpressing cyclin E is relieved by CCT deficiency, which is associated with reduced cyclin E protein levels and histone H1 kinase activity. (A) Growth on galactose plates at 25 or 35°C of wild-type (CCT2) and temperature-sensitive (cct2ts) yeast CCTβ cells transformed with either the cyclin E-expressing plasmid YCptG3(M)E or the insertless vector YCptG3(M) (31). (B) Effect of CCTβ mutation on cyclin E expression. CCT2 and cct2ts cells carrying either the cyclin E-expressing plasmid (E) or the vector (V) were grown at 35°C for 3 h in sucrose medium supplemented with 2% galactose and whole-cell RNA, and extracts were prepared. RNA was analyzed by Northern blotting with a cyclin E probe and an actin probe (lanes 1 to 4). Protein extracts were analyzed by Western blotting with an anti-cyclin E monoclonal antibody and a PSTAIRE monoclonal antibody for Cdc28p (lanes 5 to 8). Cyclin E-associated kinase activity was assayed by precipitation of extracts with the anti-cyclin E monoclonal antibody and kinase reactions with [γ-32P]ATP and histone H1 (lanes 9 to 12). (C) Cyclin E physically interacts with endogenous yeast CCT. Yeast cells expressing Cct2 bearing the Myc epitope (14) were induced for 2 h to express cyclin E (lanes 1 and 4). A cell extract was prepared, immunoprecipitated with anti-Myc antibody (Santa Cruz), and processed for Western blot analysis using anti-cyclin E monoclonal antibody. Protein extracts from cells expressing Cct1 bearing the influenza virus hemagglutinin epitope (14) after induction of cyclin E (lanes 2 and 5), and from cells expressing Cct2-Myc but lacking cyclin E (lanes 3 and 6), were also prepared and processed as negative controls.