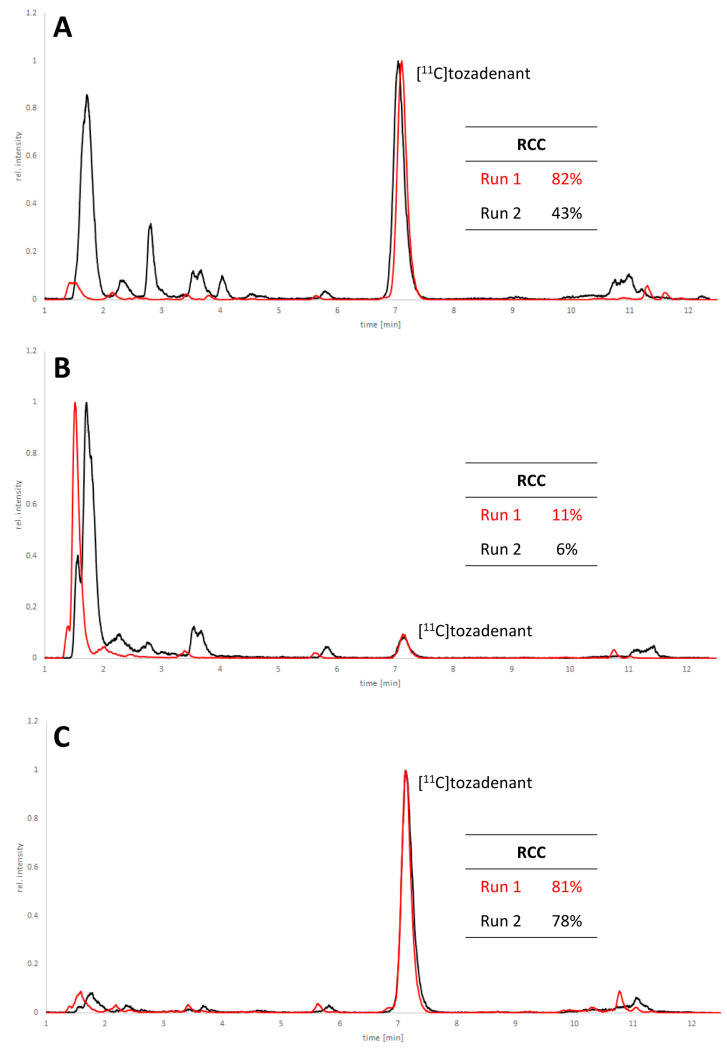
Figure 8.
HPLC chromatograms of crude reaction mixtures obtained by 11C-methylation of precursor 12 with [11C]MeI in DMF (A), DMSO (B) or DMA (C) followed by de-protection of the radiolabeled intermediate. Two reactions were performed in close succession with each solvent (indicated in red and black) and all signals were normalized to the highest peak in the corresponding chromatogram. Reaction conditions: (i) Radiomethylation: precursor (2 µmol), 1 N NaOHaq (4 µL), solvent (300 µL), 5 min, 60 °C; (ii) Deprotection: 37% HCl (150 µL), 5 min, 100 °C. HPLC conditions: Column: MultoKrom® 100-5 C18 250 × 4.6 mm; eluent: 0–10 min: 32.5% MeCNaq + 0.2% HCOOH, 10–15 min: 80% MeCNaq + 0.2% HCOOH; flow rate: 1.5 mL/min.