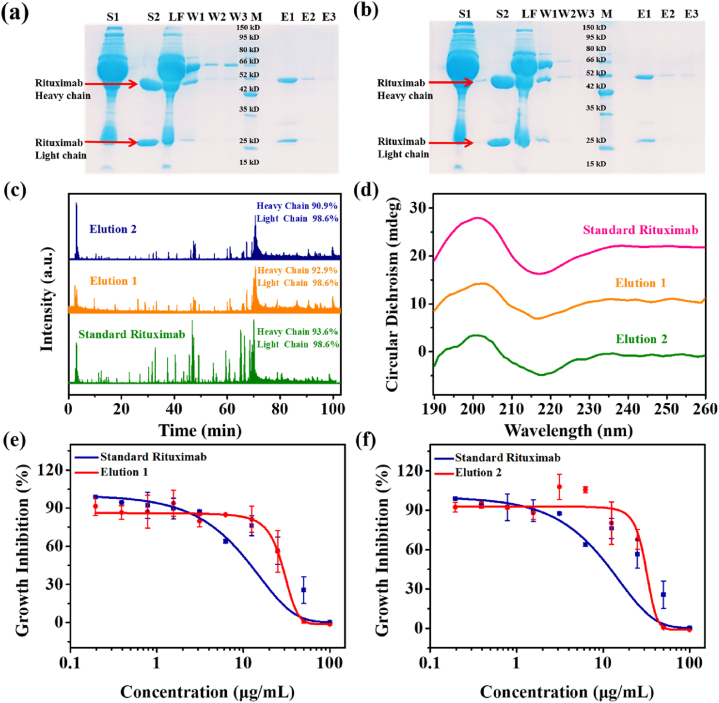
Figure 6.
Enrichment process of rituximab in spiked human serum. (a) SDS-PAGE results of rituximab enrichment on Fe3O4@NiFe LDH@HN19 from 10-fold diluted spiked human serum. (b) SDS-PAGE results of rituximab enrichment on Fe3O4@NiFe LDH@HE24 from 10-fold diluted spiked human serum. S1, feedstock; S2, standard rituximab; LF, loading fractions; W1‒W3, washing fractions; M, marker; E1‒E3, elution fractions. Stain of protein lane was achieved with Coomassie blue staining solution. (c) Mass spectra and sequence coverages of standard rituximab and the eluted protein from Fe3O4@NiFe LDH@HN19 and Fe3O4@NiFe LDH@HE24. (d) Circular dichromatic (CD) spectra of standard rituximab and the eluted protein from Fe3O4@NiFe LDH@HN19 and Fe3O4@NiFe LDH@HE24. Bioactivity analysis (cytotoxicity assay) of standard rituximab and the eluted protein from Fe3O4@NiFe LDH@HN19 (e) or from Fe3O4@NiFe LDH@HE24 (f).