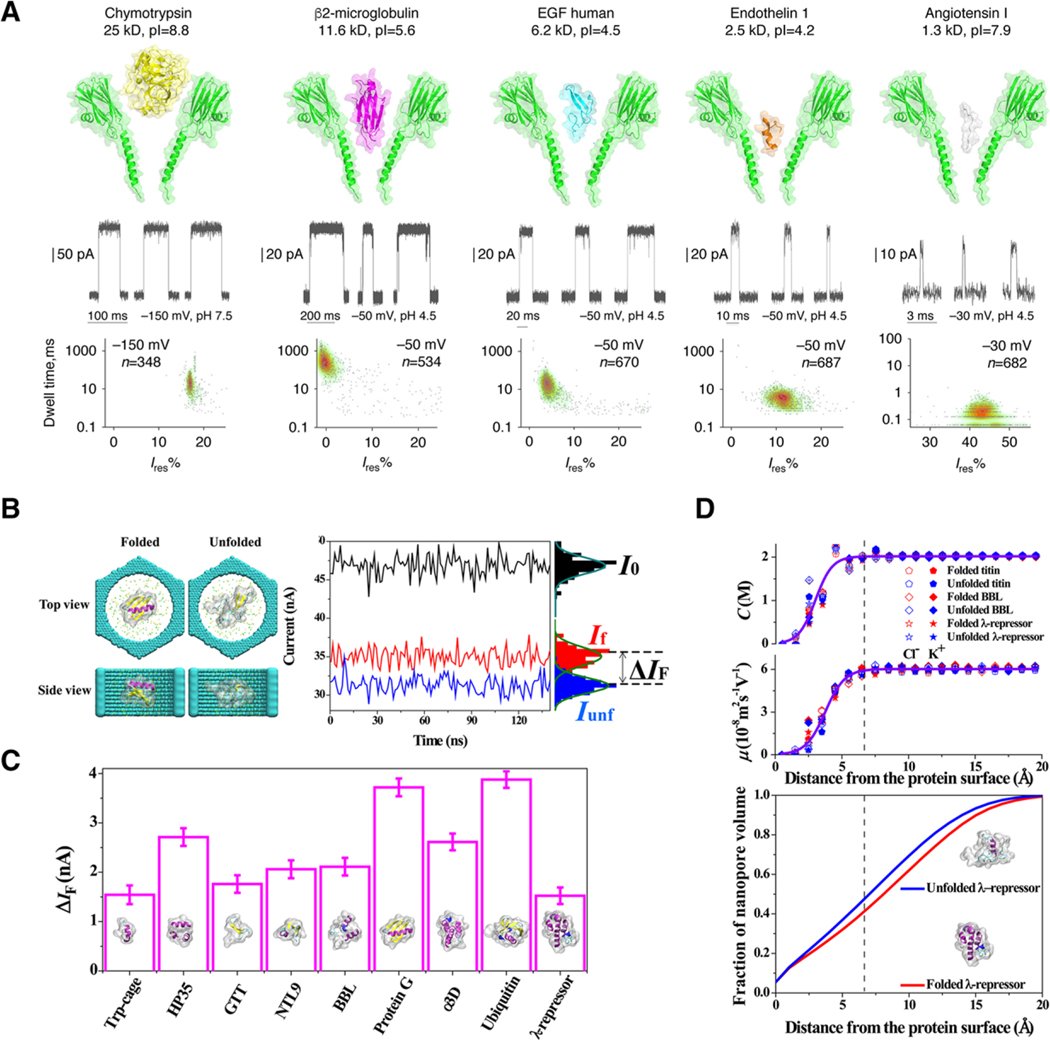
Figure 2.
Nanopore characterization of full-length proteins provides unique “spectral” fingerprints for size, chemistry and folded state. A) The flexible FraC pore can be used as a sensor for a wide range of proteins in part due to the conical structure of its transmembrane domain. Proteins are driven into the pore by electroosmotic forces and produce blockades characteristic of the size and depth of the protein partition. Reproduced under the terms and conditions of the Creative Commons Attribution license 4.0.[51] Copyright 2017, the authors, published by Springer Nature. B) Molecular dynamic simulations suggest that discrete folding states can be readily resolved in solid-state nanopores. C) The folded proteins create current blockades based on the hydrodynamic size of the protein, as it is oriented inside the pore. Error bars were calculated from the standard error of the current over 150 ns in 1.2 ns segments. D) Volume exclusion is calculated as a gradient that extends from the “surface” of the protein, which alters the mobility of ions inside the pore leading to a clear signal based on the secondary structure of the protein in folded and unfolded states. Reproduced with permission.[60] Copyright 2017, American Chemical Society.