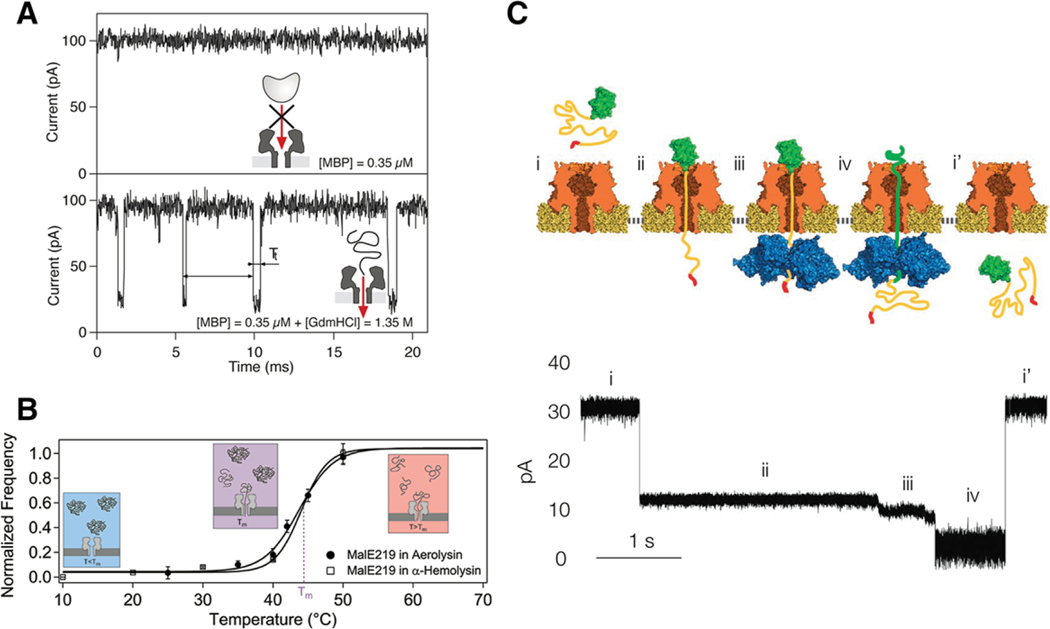
Figure 4.
Examples of protein unfolding experiments outside and inside the nanopore. A) Denaturing MBP with Gdm-HCl unfolds the protein outside the pore while maintaining the structural integrity of the αHL pore. The unfolded protein threads into the pore yielding numerous current blockades. B) Mutated MBP can also be unfolded with heat. In a similar fashion as the chemical denaturing, the protein unfolds outside the pore and leads to sizable current blockades. The relative frequency of blockades correlates with the unfolding population for both αHL and aerolysin pores. C) Unfolding has also been reported within the pore volume as was the case for the ubiquitin-like protein Smt3. An ssrA tag was attached to the Smt3 as a means for capture by a ClpX unfoldase on the trans-side of an αHL pore. The molecular motor unwinds the protein across the pore volume, which yields several substates corresponding to different stages of the unfolding process. Reproduced with permission.[71,72,78] Copyright 2007, American Physical Society; Copyright 2012, American Chemical Society; Copyright 2013, Springer Nature.