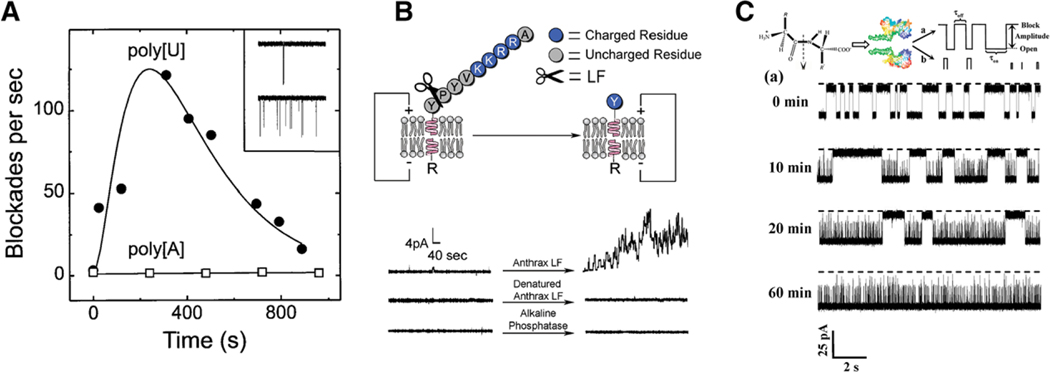
Figure 7.
Nanopore-based detection of protease activity. A) Early efforts demonstrated specific hydrolysis of polyU and not polyA with the addition of ribonuclease A through the detected increase of blockade events. Reproduced with permission.[5] Copyright 1996, The National Academy of Sciences of the USA. B) Binding a specific peptide sequence to the cis-side of the gramicidin A pore yielded clear current fluctuations upon cleavage with the lethal factor (LF) protein. This demonstrates the possibility of developing sensitive and rapid sensors for numerous transmembrane forming toxins. C) Trypsin-based cleavage of the A-β(10–20) peptide is easily detected with the onset of two distinct, shorter-lived, blockade states. Detection in this case was performed with a mutated form of the αHL pore (M113F)7. B,C) are reproduced with permission.[105,106] Copyright 2009, American Chemical Society.