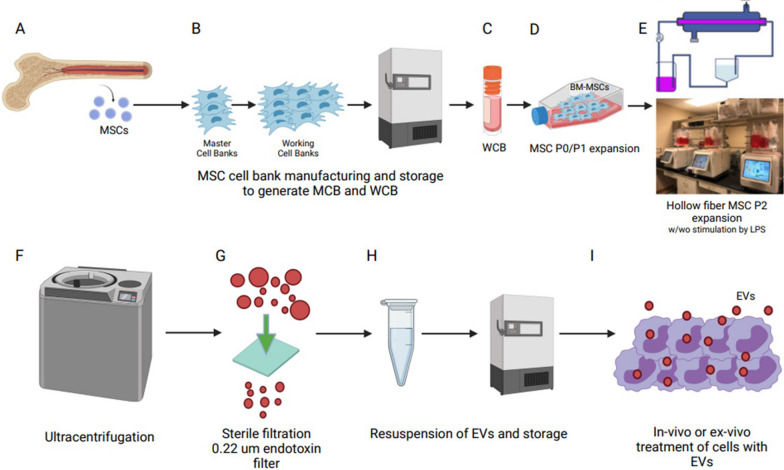
Fig. 1.
Proposed GMP manufacturing platform for MSC-EV production. A Mesenchymal stromal cells (MSCs) isolated from bone marrow (BM) should be characterized and qualified before B making a master cell bank (MCB), C followed by an expansion to generate multiple working cell banks (WCB). D Early expansion in flasks (Passage P0–P1) is followed by E expansion in a closed system bioreactor (P2) in serum free media. F EVs may be isolated directly with differential ultracentrifugation steps or concentrated beforehand using tangential flow filtration (TFF). G Resuspension of the EV pellet followed by sterile filtration (0.22 u) possibly with endotoxin removing capability. H The final EV testing and monitored storage with a consistent quality control (QC) strategy is needed to fulfill regulatory requirements for product release for I in vivo or ex vivo clinical testing. Created with Biorender.com