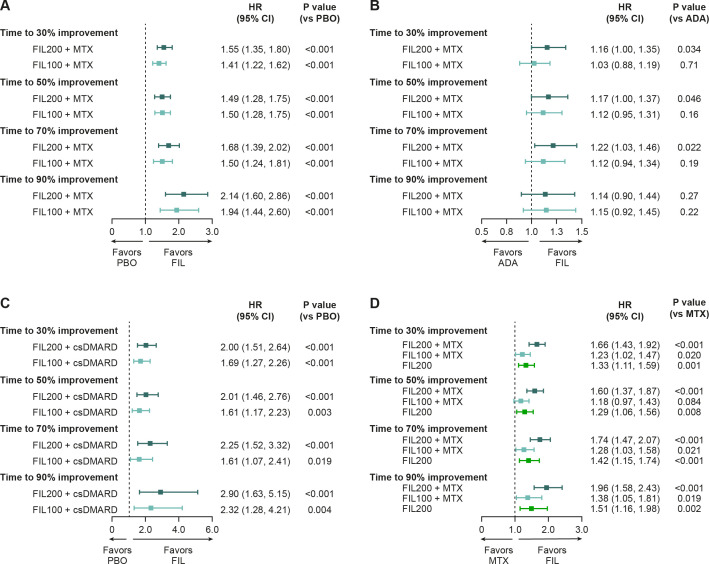
Figure 3.
Time to achieve pain improvement: stratified HR comparison between filgotinib and (A) placebo and (B) adalimumab in FINCH 1, (C) filgotinib and placebo in FINCH 2, and (D) filgotinib and methotrexate in FINCH 3. The time to event was defined as the time period (weeks) between the first dosing date and the first occurrence of the event of interest. If no event was observed during the study, the patient was censored at the latest visit. Subjects with baseline value of 0 or missing data were excluded from analysis. For FINCH 1, efficacy data through week 52 were included for the filgotinib and adalimumab groups; efficacy data through week 24 were included for the placebo group. HRs for the treatment groups were generated from a Cox regression model, stratified by geographic region and presence of anti-CCP antibodies or RF at screening (and prior exposure to bDMARDs for FINCH 1 and 2). P values were obtained from a log-rank test with the same stratification factors. ADA, adalimumab; anti-CCP, anti-cyclic citrullinated peptide; bDMARD, biologic disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; FIL100/200, filgotinib 100/200 mg; MTX, methotrexate; PBO, placebo; RF, rheumatoid factor.