Figure 2.
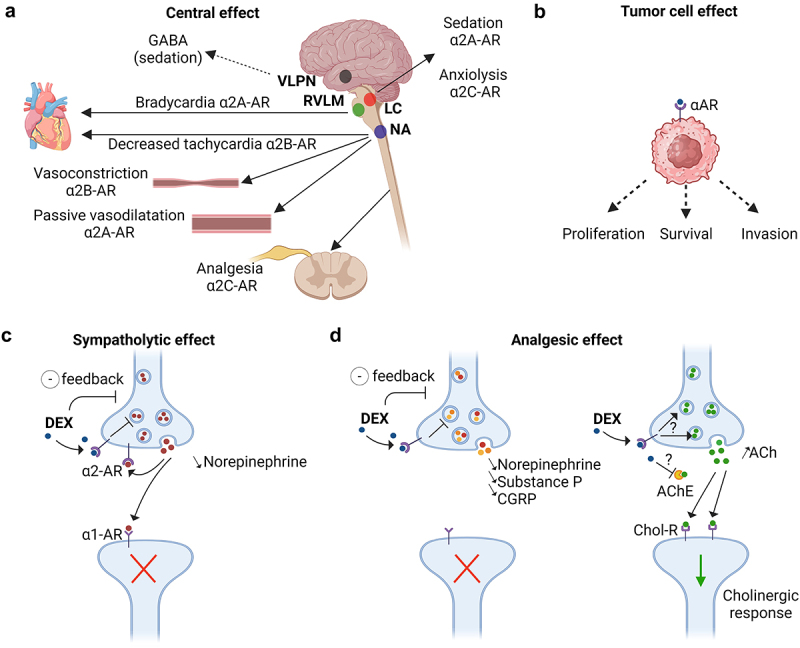
Sympatholytic and analgesic effects of dexmedetomidine through the α2-adrenoceptors.
(a, b, c) α2-adrenoceptors (α2-AR) are composed of four subtypes: α2A, α2B, α2C, and α2D. By acting on α2A-AR and α2C-AR in the locus coeruleus (LC), dexmedetomidine (DEX) decreases the release of norepinephrine from presynaptic neurons inducing sedative and anxiolytic effects. DEX could also disinhibit the ventrolateral preoptic nucleus (VLPN) promoting the release of GABA, which in turn suppresses the tuberomammillary nucleus involved in arousal. The sympatholytic action of DEX on α2A-AR in the rostral ventrolateral medulla (RVLM) and on α2B-AR in the nucleus ambiguous (NA) decreases the heart rate. On the vessels, DEX induces a transient vasoconstriction through α2B-AR, while the link to α2A-AR rather triggers a vasodilatation leading to hypotension. Moreover, DEX was described to act on α-ARs located on the surface of cancer cells, thereby exerting potential both pro- or antitumor effects. (d) DEX exhibits its analgesic properties by binding to the α2A-AR receptors in the dorsal horn of the spinal cord thus decreasing the release of nociceptive molecules such as norepinephrine, substance P and calcitonin gene-related protein (CGRP). In addition, DEX reduces neuropathic pain-induced inflammation and hyperalgesia by increasing the rate of acetylcholine (ACh) and cholinergic signaling through cholinergic receptors (Chol-R). The exact mechanism by which DEX increases the level of ACh, either through α2-AR mediated positive feedback or by inhibiting acetylcholinesterase (AChE) in the synaptic cleft, is unclear. Created with https://www.BioRender.com
