Figure 1.
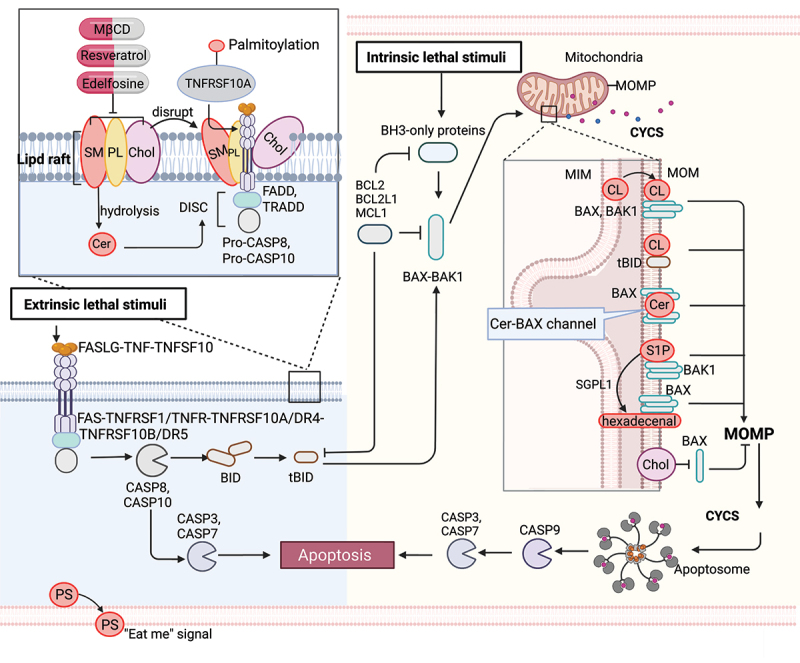
Lipid mechanism in apoptosis. Under intrinsic lethal stimuli, activated BAX or BAK1 oligomerizes and forms pores in the mitochondrial membrane, resulting in mitochondrial outer membrane permeabilization (MOMP) and the subsequent release of CYCS/cytochrome c into the cytosol. During this process, cardiolipin (CL) translocates to the mitochondrial outer membrane (MOM) and acts as a binding site for truncated BID (tBID), BAX or BAK1, triggering pore formation. Furthermore, mitochondrial ceramide (Cer) is necessary for BAX oligomerization. Cer and activated BAX together contribute to the formation of stable pores known as the ceramide channel (Cer-BAX channel). Additionally, sphingosine-1-phosphate (S1P) is degraded by SGPL1/S1P lyase to form hexadecenal. S1P and hexadecenal specifically cooperate with BAK1 and BAX, respectively. Conversely, cholesterol (Chol) accumulation in the mitochondrial membrane inhibits MOMP. Under extrinsic lethal stimuli, activated CASP8 can induce apoptosis directly by activating CASP3 or CASP7 or indirectly through the activation of the BH3-only protein BID, leading to the intrinsic apoptotic pathway. Lipid raft-disrupting agents such as methyl-beta cyclodextrin (MβCD), resveratrol, and edelfosine can trigger apoptosis. Cer, a hydrolyzed product of sphingomyelin (SM), induces coalescence of elementary rafts and enhances DISC formation, thereby amplifying FAS signaling. Furthermore, palmitoylation of TNFRSF10A/DR4 promotes TNFSF10/TRAIL-mediated apoptotic signaling. Phosphatidylserine (PS) exposure serves as an “eat me” signal for apoptotic cell clearance. [change TRAIL to “TNFSF10”.
