Abstract
Introduction
GATA3 is a transcription factor involved in epithelial cell differentiation. GATA3 immunostaining is used as a diagnostic marker for breast and urothelial cancer but can also occur in other neoplasms.
Methods
To evaluate GATA3 in normal and tumor tissues, a tissue microarray containing 16,557 samples from 131 different tumor types and subtypes and 608 samples of 76 different normal tissue types was analyzed by immunohistochemistry.
Results
GATA3 positivity was found in 69 different tumor types including 23 types (18%) with at least one strongly positive tumor. Highest positivity rates occurred in noninvasive papillary urothelial carcinoma (92–99%), lobular carcinoma (98%), carcinoma of no special type of the breast (92%), basal cell carcinoma of the skin (97%), invasive urothelial carcinoma (73%), T-cell lymphoma (23%), adenocarcinoma of the salivary gland (16%), squamous cell carcinoma of the skin (16%), and colorectal neuroendocrine carcinoma (12%). In breast cancer, low GATA3 staining was linked to high pT stage (p = 0.03), high BRE grade (p < 0.0001), HER2 overexpression (p = 0.0085), estrogen and progesterone receptor negativity (p < 0.0001 each), and reduced survival (p = 0.03).
Conclusion
Our data demonstrate that GATA3 positivity can occur in various tumor entities. Low levels of GATA3 reflect cancer progression and poor patient prognosis in breast cancer.
Keywords: GATA3, Tissue microarray, Immunohistochemistry, Human carcinomas
Introduction
The GATA3 protein is a transcription factor which is critical for the embryonic development of various tissues including the parathyroid gland and the kidney [1]. GATA3 plays a role in the luminal differentiation of breast epithelium [2, 3], development of the collecting system of the kidney and the urothelium [4, 5, 6], and trophoblastic differentiation [7, 8]. In lymphoid cells, GATA3 regulates the expression of a wide range of biologically and clinically important genes which impact inflammatory and humoral immune response [9]. GATA3 is required for the formation of T helper (Th) cells, especially Th2 cells which are pivotal for the development of allergic and humoral immune response [9, 10, 11]. GATA3 also plays a role in cancer biology. It is one of the most commonly mutated genes in breast cancer where it plays a role in estrogen and androgen receptor signaling [12, 13, 14, 15].
Normal tissue analyses have revealed GATA3 expression in breast, kidney, urothelium, parathyroid, placenta, skin, salivary gland, and lymphatic organs [16, 17, 18, 19, 20, 21, 22, 23]. Because of its selective expression in a rather limited number of tissues, GATA3 is used as an immunohistochemical marker in diagnostic pathology, where GATA3 is mainly used for supporting the distinction of breast or urothelial cancer from other diagnostic options [6, 24]. In the literature, the positivity rates range from 70 to 100% for lobular breast cancer [25, 26, 27, 28], from 30% to 100% for breast cancer of no special type (NST) [26, 29, 30, 31, 32, 33], and from 45–99% for muscle-invasive urothelial carcinoma [34, 35, 36, 37, 38, 39, 40, 41, 42, 43]. Discrepant data (also) exist for various other tumor types. For example, GATA3 positivity has been described in 0–44% of gastric adenocarcinoma [44, 45, 46, 47], 0–74% of non-small cell lung adenocarcinoma [27, 48, 49, 50], 0–76% of clear cell papillary renal cell carcinoma [6, 17, 51], 9–44% of anaplastic thyroid carcinoma [33, 52], and 13–57% of serous endometrial carcinomas [53, 54]. These conflicting data are probably caused by the use of different antibodies, immunostaining protocols, and criteria to determine GATA3 positivity in these studies.
To better understand the prevalence and significance of GATA3 expression in cancer, a comprehensive study analyzing a large number of neoplastic and non-neoplastic tissues under highly standardized conditions is desirable. Therefore, GATA3 expression was analyzed in more than 16,000 tumor tissue samples from 131 different tumor types and subtypes as well as 76 non-neoplastic tissue categories by immunohistochemistry (IHC) in a tissue microarray (TMA) format in this study.
Materials and Methods
Tissue Microarrays
Our normal tissue TMA was composed of 8 samples from 8 different donors for each of 76 different normal tissue types (608 samples on one slide). The tumor TMAs contained a total of 16,557 primary tumors from 131 tumor types and subtypes. Detailed histopathological and molecular data were available for 1,475 cancers of the breast. Clinical follow-up data were accessible from 877 breast cancer patients with a median follow-up time of 49 months. The composition of normal and tumor TMAs is described in the results section. All samples were from the archives of the Institutes of Pathology, University Hospital of Hamburg, Germany; the Institute of Pathology, Clinical Center Osnabrueck, Germany; and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. The TMA manufacturing process was described earlier in detail [55, 56]. In brief, one tissue spot (diameter: 0.6 mm) was transmitted from a representative tumor containing donor block in an empty recipient paraffin block. The use of archived remnants of diagnostic tissues for TMA manufacturing, their analysis for research purposes, and patient data were according to local laws (HmbKHG, §12), and analysis had been approved by the Local Ethics Committee (Ethics Commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Immunohistochemistry
Freshly cut TMA sections were immunostained on one day and in one experiment. Two different primary antibodies were used for GATA3 detection: MSVA-450M (mouse monoclonal; cat.# 2980-450M; MS Validated Antibodies GmbH, Hamburg, Germany) and clone L50-823 (mouse monoclonal, cat.# MSK100-05, Zytomed Systems, Berlin, Germany). For both antibodies, slides were deparaffinized and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121°C in a pH 7.8 buffer. MSVA-450M was applied at 37°C for 60 min at a dilution of 1:50. L50-823 was applied at 37°C for 60 min at a dilution of 1:300. Bound antibody was then visualized using the Envision Kit (Dako, Glostrup, Denmark) according to the manufacturer's directions. All tissues were manually scored by one pathologist (VR). Questionable cases were discussed with the supervisor of the study (FJ). For normal tissues, staining intensity was recorded as 0, 1+, 2+, and 3+. For tumor tissues, the percentage of positive neoplastic cells was estimated, and the staining intensity was recorded as 0, 1+, 2+, and 3+. For statistical analyses, the tumor staining results were categorized into four groups as described before [57]. Tumors without any staining were considered negative. Tumors with 1+ staining intensity in ≤70% of tumor cells or 2+ intensity in ≤30% of tumor cells were considered weakly positive. Tumors with 1+ staining intensity in >70% of tumor cells, or 2+ intensity in 31–70%, or 3+ intensity in ≤30% of tumor cells were considered moderately positive. Tumors with 2+ intensity in >70% or 3+ intensity in >30% of tumor cells were considered strongly positive. Multiplex fluorescence IHC and digital image analysis were performed on prostate cancer tissue to assign GATA3 expression in lymphocytes to specific cell subsets. For details, see online supplementary methods (see www.karger.com/doi/10.1159/000527382 for all online suppl. material).
Statistics
Statistical calculations were performed with JMP 14 software (SAS Institute Inc., NC, USA). Contingency tables and the χ2 test were performed to search for associations between GATA3 and tumor phenotype. Survival curves were calculated according to Kaplan-Meier. The log-rank test was applied to detect significant differences between groups. A p value of ≥0.05 was considered as statistically significant.
Results
Technical Issues
A total of 13,093 (79%) of 16,557 tumor samples were interpretable in our TMA analysis. Noninterpretable samples demonstrated lack of unequivocal tumor cells or loss of the tissue spot during technical procedures. A sufficient number of samples of each normal tissue type were evaluable.
GATA3 in Normal Tissues
By using MSVA-450M, nuclear GATA3 immunostaining was seen in urothelium (+++), squamous epithelium of the skin (+++; superficial cell layers > basal cell layers), hair follicles (+++), sebaceous glands (++), parathyroid gland (+++), trophoblastic cells (+++), chorion cells (+++), and amnion cells (+) of the placenta, collecting ducts (++; not all) and glomerular podocytes (++) of the kidney, seminal vesicle epithelium (+++), tall columnar cells and basal cells (++) of the epididymis, a fraction of the luminal cells of breast glands (+++), basal cells in the prostate (+; not always visible), glandular cells (especially mucinous) of salivary glands (+ - ++), and a fraction of lymphocytes, most prominently in the thymus (++). Multiplex fluorescence IHC identified the positive lymphocytes as Th cells (online suppl. Fig. 1). A faint cytoplasmic GATA3 staining was seen in gastric glands and goblet cells of respiratory epithelium, most likely representing nonspecific background staining. Representative images of GATA3 in normal tissues analyzed with MSVA-450M are shown in Figure 1. GATA3 immunostaining was absent in gastrointestinal epithelium, gallbladder, liver, pancreas, lung, fallopian tube, endometrium, and endocervical glands of the uterus, ovary, nonkeratinizing squamous epithelium from various sites, mesenchymal tissues, pituitary gland, and the brain. All cell types identified as positive by MSVA-450M were confirmed by L50-823 staining (online suppl. Fig. 2).
Fig. 1.
GATA3 immunostaining of normal tissues. The panels show a strong nuclear GATA3 positivity of predominantly suprabasal cells in the epidermis of the skin (a), urothelial cells of all layers (b), podocytes and collecting duct cells of the kidney (c), luminal cells of the breast (d), trophoblastic cells of the placenta (e), and epithelial cells of the parathyroid gland (f).
GATA3 in Cancer
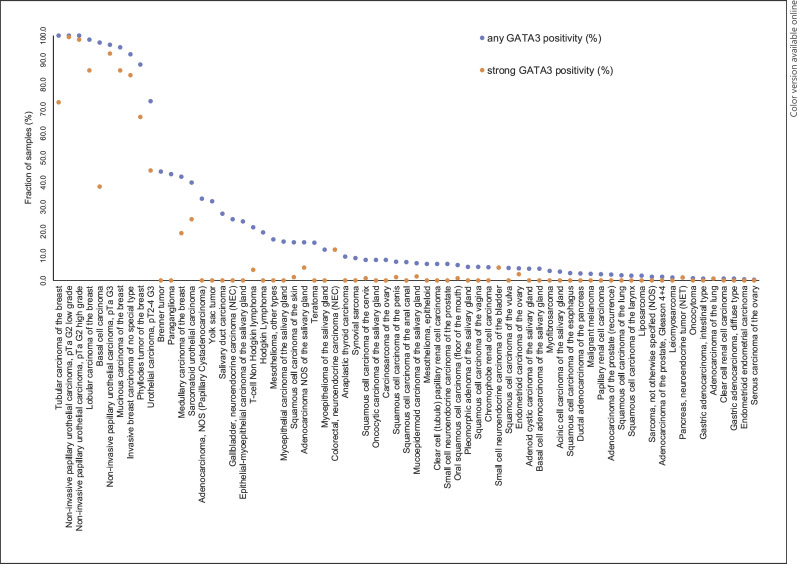
Positive GATA3 immunostaining was detectable in 2,567 (19.6%) of the 13,093 analyzable tumors, including 315 (2.4%) with weak, 285 (2.2%) with moderate, and 1,967 (15%) with strong immunostaining. Overall, 69 (53%) of 131 tumor categories showed detectable GATA3 expression with 23 (18%) tumor categories including at least one case with strong positivity (Table 1). Representative images of GATA3-positive tumors are shown in Figure 2. The highest rate of positive staining and the highest levels of expression were found in various subtypes of breast and urinary bladder neoplasms as well as in basal cell carcinoma of the skin. At lower frequency and often at lower intensity, GATA3 immunostaining could be seen in various categories of salivary gland tumors, neuroendocrine tumors (NETs) as well as in squamous cell carcinomas or tumors containing squamous cell elements such as endometroid carcinomas. In the cases of phyllodes tumor, myoepithelial tumors, and other biphasic tumors, always the epithelial component was positive. A graphical representation of a ranking order of GATA3-positive and strongly positive tumors is given in Figure 3. In a cohort of 1,079 breast cancers of NST, low or absent GATA3 immunostaining was linked to advanced pT stage (p = 0.032), high BRE grade (p < 0.0001), HER2 overexpression (p = 0.0085), estrogen and progesterone receptor negativity (p < 0.0001 each; Table 2) as well as reduced overall survival (p = 0.0304; Fig. 4).
Table 1.
GATA3 immunostaining in human tumors
| Tumor entity | On TMA, n | GATA3 immunostaining |
|||||
|---|---|---|---|---|---|---|---|
| interpr, n | negative (%) | weak (%) | moderate (%) | strong (%) | |||
| Tumors of the skin | Pilomatricoma | 35 | 31 | 100.0 | 0.0 | 0.0 | 0.0 |
| Basal cell carcinoma | 88 | 68 | 2.9 | 11.8 | 47.1 | 38.2 | |
| Benign nevus | 29 | 22 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the skin | 90 | 77 | 84.4 | 14.3 | 0.0 | 1.3 | |
| Malignant melanoma | 46 | 38 | 97.4 | 2.6 | 0.0 | 0.0 | |
| Merkel cell carcinoma | 46 | 40 | 100.0 | 0.0 | 0.0 | 0.0 | |
|
| |||||||
| Tumors of the head and neck | Squamous cell carcinoma of the larynx | 109 | 103 | 98.1 | 1.9 | 0.0 | 0.0 |
| Squamous cell carcinoma of the pharynx | 60 | 59 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Oral squamous cell carcinoma (floor of the mouth) | 130 | 127 | 93.7 | 3.9 | 1.6 | 0.8 | |
| Pleomorphic adenoma of the parotid gland | 50 | 46 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Warthin tumor of the parotid gland | 104 | 96 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adenocarcinoma, NOS (papillary cystadenocarcinoma) | 14 | 12 | 66.7 | 8.3 | 25.0 | 0.0 | |
| Salivary duct carcinoma | 15 | 11 | 72.7 | 18.2 | 9.1 | 0.0 | |
| Acinic cell carcinoma of the salivary gland | 181 | 142 | 96.5 | 2.8 | 0.7 | 0.0 | |
| Adenocarcinoma NOS of the salivary gland | 109 | 77 | 84.4 | 9.1 | 1.3 | 5.2 | |
| Adenoid cystic carcinoma of the salivary gland | 180 | 104 | 95.2 | 3.8 | 1.0 | 0.0 | |
| Basal cell adenocarcinoma of the salivary gland | 25 | 21 | 95.2 | 0.0 | 4.8 | 0.0 | |
| Basal cell adenoma of the salivary gland | 101 | 88 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Epithelial-myoepithelial carcinoma of the salivary gland | 53 | 50 | 76.0 | 12.0 | 12.0 | 0.0 | |
| Mucoepidermoid carcinoma of the salivary gland | 343 | 257 | 93.0 | 4.3 | 1.2 | 1.6 | |
| Myoepithelial carcinoma of the salivary gland | 21 | 19 | 84.2 | 5.3 | 10.5 | 0.0 | |
| Myoepithelioma of the salivary gland | 11 | 8 | 87.5 | 0.0 | 12.5 | 0.0 | |
| Oncocytic carcinoma of the salivary gland | 12 | 12 | 91.7 | 8.3 | 0.0 | 0.0 | |
| Polymorphous adenocarcinoma, low grade, of the salivary gland | 41 | 31 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Pleomorphic adenoma of the salivary gland | 53 | 36 | 94.4 | 5.6 | 0.0 | 0.0 | |
|
| |||||||
| Tumors of the lung, pleura, and thymus | Adenocarcinoma of the lung | 196 | 138 | 99.3 | 0.0 | 0.0 | 0.7 |
| Squamous cell carcinoma of the lung | 80 | 50 | 98.0 | 2.0 | 0.0 | 0.0 | |
| Small cell carcinoma of the lung | 16 | 11 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Mesothelioma, epithelioid | 39 | 30 | 93.3 | 6.7 | 0.0 | 0.0 | |
| Mesothelioma, other types | 76 | 54 | 83.3 | 11.1 | 5.6 | 0.0 | |
|
| |||||||
| Tumors of the female genital tract | Squamous cell carcinoma of the vagina | 78 | 55 | 94.5 | 3.6 | 1.8 | 0.0 |
| Squamous cell carcinoma of the vulva | 130 | 118 | 94.9 | 5.1 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the cervix | 128 | 119 | 91.6 | 5.9 | 1.7 | 0.8 | |
| Endometrioid endometrial carcinoma | 236 | 222 | 99.5 | 0.5 | 0.0 | 0.0 | |
| Endometrial serous carcinoma | 82 | 66 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Carcinosarcoma of the uterus | 48 | 38 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Endometrial carcinoma, high grade, G3 | 13 | 13 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Endometrial clear cell carcinoma | 8 | 7 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Endometrioid carcinoma of the ovary | 110 | 82 | 95.1 | 2.4 | 0.0 | 2.4 | |
| Serous carcinoma of the ovary | 559 | 443 | 99.8 | 0.2 | 0.0 | 0.0 | |
| Mucinous carcinoma of the ovary | 96 | 62 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Clear cell carcinoma of the ovary | 50 | 37 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Carcinosarcoma of the ovary | 47 | 36 | 91.7 | 8.3 | 0.0 | 0.0 | |
| Brenner tumor | 9 | 9 | 55.6 | 0.0 | 44.4 | 0.0 | |
|
| |||||||
| Tumors of the breast | Invasive breast carcinoma of NST | 1,345 | 1,137 | 7.7 | 3.6 | 4.9 | 83.8 |
| Lobular carcinoma of the breast | 293 | 232 | 1.7 | 3.4 | 9.1 | 85.8 | |
| Medullary carcinoma of the breast | 26 | 26 | 57.7 | 7.7 | 15.4 | 19.2 | |
| Tubular carcinoma of the breast | 27 | 22 | 0.0 | 9.1 | 18.2 | 72.7 | |
| Mucinous carcinoma of the breast | 58 | 42 | 4.8 | 4.8 | 4.8 | 85.7 | |
| Phyllodes tumor of the breast | 50 | 42 | 11.9 | 7.1 | 14.3 | 66.7 | |
|
| |||||||
| Tumors of the digestive system | Adenomatous polyp, low-grade dysplasia | 50 | 46 | 100.0 | 0.0 | 0.0 | 0.0 |
| Adenomatous polyp, high-grade dysplasia | 50 | 46 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adenocarcinoma of the colon | 1,882 | 1,587 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Gastric adenocarcinoma, diffuse type | 176 | 145 | 99.3 | 0.7 | 0.0 | 0.0 | |
| Gastric adenocarcinoma, intestinal type | 174 | 137 | 99.3 | 0.7 | 0.0 | 0.0 | |
| Gastric adenocarcinoma, mixed type | 62 | 56 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adenocarcinoma of the esophagus | 83 | 51 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the esophagus | 76 | 33 | 97.0 | 3.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the anal canal | 89 | 80 | 92.5 | 3.8 | 3.8 | 0.0 | |
| Cholangiocarcinoma | 113 | 105 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Hepatocellular carcinoma | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Ductal adenocarcinoma of the pancreas | 612 | 430 | 97.2 | 1.9 | 0.9 | 0.0 | |
| Pancreatic/ampullary adenocarcinoma | 89 | 71 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Acinar cell carcinoma of the pancreas | 16 | 14 | 100.0 | 0.0 | 0.0 | 0.0 | |
| GIST | 50 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | |
|
| |||||||
| Tumors of the urinary system | Noninvasive papillary urothelial carcinoma, pTa G2 low grade | 177 | 147 | 0.0 | 0.0 | 0.7 | 99.3 |
| Noninvasive papillary urothelial carcinoma, pTa G2 high grade | 141 | 117 | 0.0 | 0.0 | 1.7 | 98.3 | |
| Noninvasive papillary urothelial carcinoma, pTa G3 | 187 | 161 | 3.7 | 0.6 | 3.1 | 92.5 | |
| Urothelial carcinoma, pT2-4 G3 | 1,206 | 603 | 26.9 | 13.1 | 15.1 | 44.9 | |
| Small cell NEC of the bladder | 20 | 19 | 94.7 | 0.0 | 0.0 | 5.3 | |
| Sarcomatoid urothelial carcinoma | 25 | 20 | 60.0 | 5.0 | 10.0 | 25.0 | |
| Clear cell renal cell carcinoma | 857 | 578 | 99.3 | 0.2 | 0.5 | 0.0 | |
| Papillary renal cell carcinoma | 255 | 161 | 97.5 | 0.0 | 2.5 | 0.0 | |
| Clear cell (tubulo) papillary renal cell carcinoma | 21 | 15 | 93.3 | 6.7 | 0.0 | 0.0 | |
| Chromophobe renal cell carcinoma | 131 | 93 | 94.6 | 2.2 | 3.2 | 0.0 | |
| Oncocytoma | 177 | 114 | 99.1 | 0.0 | 0.9 | 0.0 | |
|
| |||||||
| Tumors of the male genital | Adenocarcinoma of the prostate, Gleason 3 + 3 | 83 | 79 | 100.0 | 0.0 | 0.0 | 0.0 |
|
| |||||||
| organs | Adenocarcinoma of the prostate, Gleason 4 + 4 | 80 | 67 | 98.5 | 0.0 | 1.5 | 0.0 |
| Adenocarcinoma of the prostate, Gleason 5 + 5 | 85 | 77 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adenocarcinoma of the prostate (recurrence) | 258 | 175 | 97.7 | 2.3 | 0.0 | 0.0 | |
| Small cell NEC of the prostate | 19 | 15 | 93.3 | 6.7 | 0.0 | 0.0 | |
| Seminoma | 621 | 608 | 99.8 | 0.0 | 0.2 | 0.0 | |
| Embryonal carcinoma of the testis | 50 | 30 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Yolk sac tumor | 50 | 31 | 67.7 | 29.0 | 3.2 | 0.0 | |
| Teratoma | 50 | 39 | 84.6 | 10.3 | 5.1 | 0.0 | |
| Squamous cell carcinoma of the penis | 80 | 79 | 92.4 | 6.3 | 0.0 | 1.3 | |
|
| |||||||
| Tumors of endocrine organs | Adenoma of the thyroid gland | 113 | 103 | 100.0 | 0.0 | 0.0 | 0.0 |
| Papillary thyroid carcinoma | 391 | 351 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Follicular thyroid carcinoma | 154 | 128 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Medullary thyroid carcinoma | 111 | 91 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Anaplastic thyroid carcinoma | 45 | 41 | 90.2 | 9.8 | 0.0 | 0.0 | |
| Adrenal cortical adenoma | 50 | 35 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adrenal cortical carcinoma | 26 | 25 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Phaeochromocytoma | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Appendix, NET | 22 | 11 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Colorectal, NET | 12 | 9 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Ileum, NET | 49 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Lung, NET | 19 | 17 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Pancreas, NET | 97 | 91 | 98.9 | 0.0 | 0.0 | 1.1 | |
| Colorectal, NEC | 12 | 8 | 87.5 | 0.0 | 0.0 | 12.5 | |
| Gallbladder, NEC | 4 | 4 | 75.0 | 25.0 | 0.0 | 0.0 | |
| Pancreas, NEC | 14 | 14 | 100.0 | 0.0 | 0.0 | 0.0 | |
|
| |||||||
| Tumors of haemato-poetic and lymphoid tissues | Hodgkin lymphoma | 103 | 92 | 80.4 | 18.5 | 1.1 | 0.0 |
| Small lymphocytic lymphoma, B-cell type (B-SLL/B-CLL) | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 | |
| DLBCL | 113 | 112 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Follicular lymphoma | 88 | 88 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Mantle cell lymphoma | 18 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Marginal zone lymphoma | 16 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| DLBCL in the testis | 16 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Burkitt lymphoma | 5 | 5 | 100.0 | 0.0 | 0.0 | 0.0 | |
|
| |||||||
| Tumors of soft tissue and bone | Tendosynovial giant cell tumor | 45 | 40 | 100.0 | 0.0 | 0.0 | 0.0 |
| Granular cell tumor | 53 | 39 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyoma | 50 | 48 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyosarcoma | 87 | 84 | 98.8 | 1.2 | 0.0 | 0.0 | |
| Li po sarcoma | 132 | 106 | 98.1 | 1.9 | 0.0 | 0.0 | |
| MPNST | 13 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Myofibrosarcoma | 26 | 26 | 96.2 | 3.8 | 0.0 | 0.0 | |
| Angiosarcoma | 73 | 58 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Angiomyolipoma | 91 | 83 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Dermatofibrosarcoma protuberans | 21 | 15 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Ganglioneuroma | 14 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Kaposi sarcoma | 8 | 3 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Neurofibroma | 117 | 81 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Sarcoma, NOS | 74 | 66 | 98.5 | 1.5 | 0.0 | 0.0 | |
| Paraganglioma | 41 | 30 | 56.7 | 33.3 | 10.0 | 0.0 | |
| Ewing sarcoma | 23 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Rhabdomyosarcoma | 6 | 6 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Schwannoma | 121 | 91 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Synovial sarcoma | 12 | 11 | 90.9 | 9.1 | 0.0 | 0.0 | |
| Osteosarcoma | 43 | 30 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Chondrosarcoma | 38 | 23 | 100.0 | 0.0 | 0.0 | 0.0 | |
GIST, gastrointestinal stromal tumor; DLBCL, diffuse large B-cell lymphoma; MPNST, malignant peripheral nerve sheath tumor; NOS, not otherwise specified.
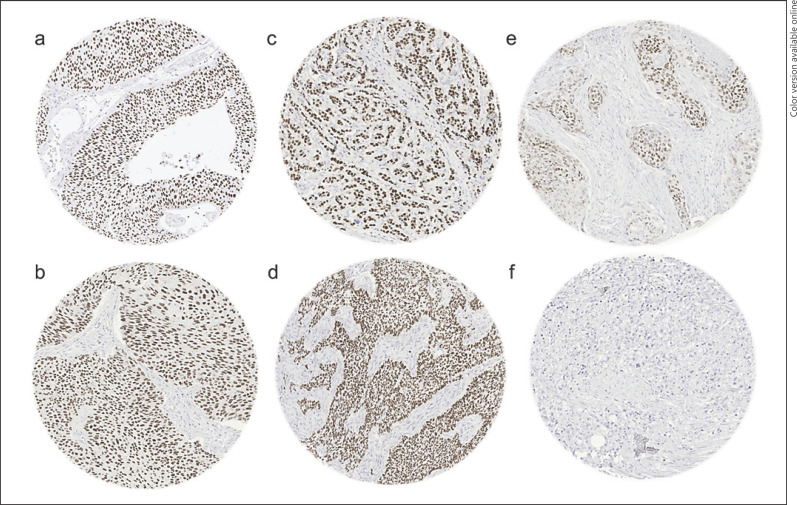
Fig. 2.
GATA3 immunostaining in cancer. The panels show a strong GATA3 positivity in cases of noninvasive (pTa) urothelial carcinoma (a), muscle-invasive urothelial carcinoma (b), invasive lobular carcinoma of the breast (c), basal cell carcinoma of the skin (d) as well as a weak to moderate GATA3 staining in a squamous cell carcinoma of the skin (e) and absence of staining in a prostatic adenocarcinoma (Gleason 5 + 5 = 10) (f).
Fig. 3.
Ranking order of GATA3 immunostaining in tumors. Both the frequency of positive cases (blue dots) and the frequency of strongly positive cases (orange dots) are shown.
Table 2.
GATA3 immunostaining and tumor phenotype in breast carcinoma of NST
| N | GATA3 immunostaining result |
|||||||
|---|---|---|---|---|---|---|---|---|
| negative (%) | weak (%) | moderate (%) | strong (%) | p value | ||||
| Breast carcinoma of NST | Primary tumor | pT1 | 566 | 4.9 | 3.2 | 3.5 | 88.3 | 0.032 |
| pT2 | 394 | 7.4 | 3.8 | 5.1 | 83.8 | |||
| pT3-4 | 83 | 13.3 | 7.2 | 6.0 | 73.5 | |||
|
|
||||||||
| Grade | G1 | 171 | 1.2 | 0.0 | 0.6 | 98.2 | <0.0001 | |
| G2 | 556 | 3.2 | 2.7 | 2.9 | 91.2 | |||
| G3 | 350 | 15.7 | 6.9 | 8.9 | 68.6 | |||
|
|
||||||||
| Regional lymph nodes | pN0 | 497 | 5.6 | 4.0 | 5.0 | 85.3 | 0.9688 | |
| pN1 | 214 | 7.0 | 3.3 | 4.7 | 85.0 | |||
| pN2 | 66 | 6.1 | 4.5 | 6.1 | 83.3 | |||
| pN3 | 56 | 7.1 | 5.4 | 8.9 | 78.6 | |||
|
|
||||||||
| HER2 status | Negative | 806 | 7.8 | 3.3 | 4.0 | 84.9 | 0.0085 | |
| Positive | 106 | 3.8 | 9.4 | 7.5 | 79.2 | |||
|
|
||||||||
| ER status | Negative | 181 | 34.3 | 17.7 | 14.9 | 33.1 | <0.0001 | |
| Positive | 688 | 0.3 | 0.6 | 1.6 | 97.5 | |||
|
|
||||||||
| PR status | Negative | 362 | 17.4 | 9.1 | 9.7 | 63.8 | <0.0001 | |
| Positive | 544 | 1.1 | 0.7 | 1.3 | 96.9 | |||
|
|
||||||||
| Triple negative | No | 720 | 1.3 | 1.9 | 2.4 | 94.4 | <0.0001 | |
| Yes | 121 | 44.6 | 18.2 | 16.5 | 20.7 | |||
Fig. 4.
GATA3 immunostaining and overall survival in no special type breast carcinoma.
Discussion
The International Working Group for Antibody Validation (IWGAV) had proposed that antibody validation for IHC on formalin fixed tissues should include a comparison with expression data obtained by another independent method or by a different antibody to the same target [58]. Our normal tissue analysis revealed nuclear GATA3 immunostaining in parathyroid gland, urothelium, squamous epithelium of the skin, placenta, kidney, seminal vesicle, prostate, breast, salivary glands, and lymphatic tissues, most prominently in the thymus. These findings almost completely match data from three independent RNA screening studies including the Human Protein Atlas (HPA) RNA seq tissue dataset [59], the FANTOM5 project [60, 61], and the Genotype-Tissue Expression (GTEx) project [62]. The only discrepancy between our data and these RNA findings is that GATA3 positivity in the vagina as suggested by GTEx and FANTOM5 is not confirmed by our analysis. As we found significant GATA3 staining in keratinizing but not in nonkeratinizing squamous epithelium and because keratinization can occur at the vaginal orifice, we assume that differences in sampling might be responsible for this disagreement. That all GATA3-positive cell types were also detected by a second independent antibody further validates our findings. That multicolor immunofluorescence identified most GATA3-positive lymphocytes in prostate cancer tissue as CD4+ Th cells is well consistent with the literature (reviewed in [9, 63]).
The data obtained by an analysis of 13,093 tumors from 131 different subtypes were largely reflective of the GATA3 expression in corresponding normal tissues. This was to be expected because it has already been shown for many other diagnostic biomarkers that expression of tissue-specific proteins is often retained in neoplasms [64, 65]. That urothelial carcinoma and breast cancer were the most commonly GATA3-positive cancers may be because the urothelium and breast glands are the most cancer susceptible organs among GATA3-positive normal tissues. We found a striking difference in the rate of GATA3 positivity between basal cell carcinoma (97% positive) and squamous cell carcinoma of the skin (16% positive). Previous studies on 14–62 samples per entity reported rather similar overall positivity rates for basal cell (90–100%) and squamous cell carcinomas (81–88%) [19, 33, 66, 67], but found marked differences in the staining intensity or fraction of stained tumor cells. For example, Mertens et al. reported strong staining in 13 of 14 GATA3-positive basal cell carcinomas but in only 4 of 21 GATA3-positive squamous cell cancers [19], and Miettinen et al. [33] mentioned strong and uniform positivity in all cells of basal cell carcinomas but a wide range (5–100%) of positive tumor cells in squamous cell cancers. It is likely that different experimental conditions account for a higher sensitivity to detect (very) low GATA3 expression in these studies. However, GATA3 was more intense in superficial than in basal cell layers of the normal skin in our study, suggesting that GATA3 may belong to these proteins that gradually enrich when cells are pushed from the basal layers through the stratum spinosum toward the outer cell layers of the skin [68]. A general role of GATA3 for a specific growth and maturation status of squamous epithelium − independent from the site of origin − is further supported by our observation that the GATA3 positivity rate was only minimally higher in squamous cell carcinomas derived from the GATA3-positive skin than in squamous cell carcinomas derived from GATA3-negative nonkeratinizing squamous epithelia.
The markedly lower rate and intensity of GATA3 positivity in salivary gland tumors as compared to breast and urothelial cancer fit to the somewhat lower GATA3 expression levels in normal salivary glands as compared to normal breast and urothelial cells. The low rate of GATA3 positivity in kidney and prostate cancer is obviously due to the rarity of malignant transformation of GATA3-positive cell types in these organs. Most renal cell carcinomas develop from the proximal or distal tubules and not from collecting ducts or even podocytes [69], and prostate neoplasms do only very rarely originate from basal cells [70].
Our data support the use of GATA3 IHC for several applications that have been earlier proposed. This includes the distinction of metastatic urothelial and breast carcinomas (often GATA3+) from other metastatic carcinomas (mostly GATA3−) [71, 72], the distinction of urothelial carcinoma (mostly GATA3+) [73] from prostatic carcinoma (mostly GATA3−) [74], and the distinction of metastatic lobular carcinoma of the breast (GATA3+) [75, 76] from gastric signet ring cell carcinoma (GATA3−) [46]. It is of note, however, that a weak or even moderate to strong GATA3 immunostaining can occasionally occur in various further clinically important tumor entities including neuroendocrine cancer (NEC), adenocarcinoma of the lung, malignant mesothelioma, germ cell tumor, renal cell carcinoma, prostate cancer, and pancreatic adenocarcinoma.
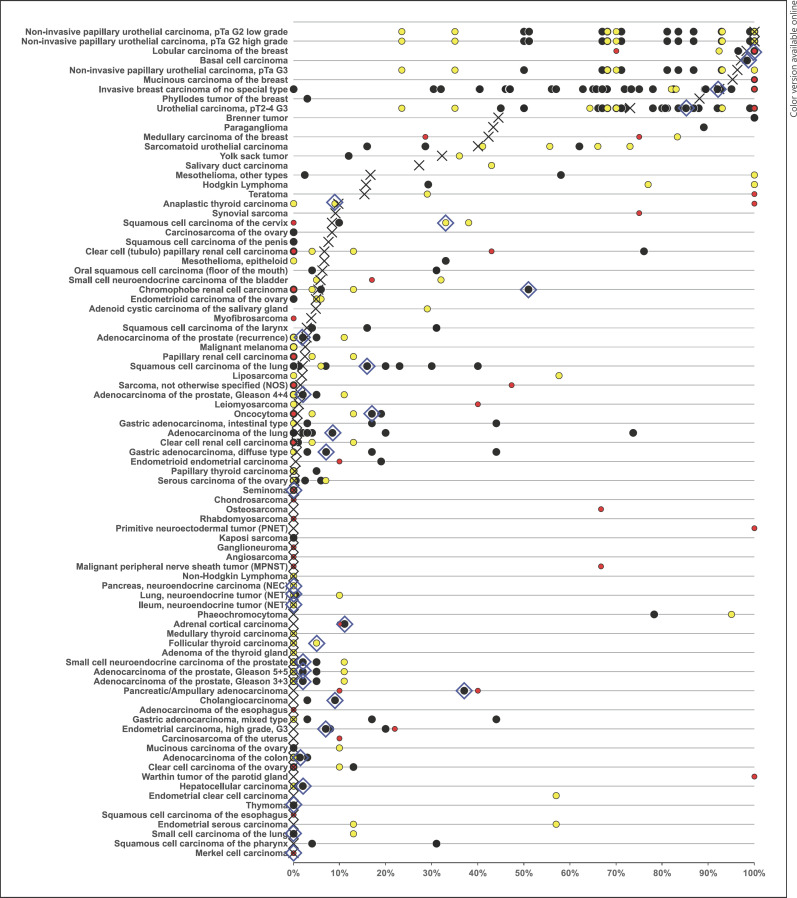
The availability of clinical and molecular data enabled us to interrogate the potential clinical significance of GATA3 expression in breast cancer. That a reduced GATA3 expression was linked to unfavorable tumor phenotype, HER2 positivity, ER/PR negativity, and poor prognosis is consistent with the literature [31, 77, 78, 79, 80, 81]. These findings are consistent with a model assuming that reduced GATA3 expression in tumors derived from GATA3-positive precursor cells reflects cellular dedifferentiation which is generally linked to unfavorable tumor phenotype and increased cancer aggressiveness. A key result of this study is a ranking order of human tumors according to the prevalence and intensity of GATA3 expression. The comparison with previously published data in the literature demonstrates that this information cannot easily be obtained from the literature given the highly discrepant data on many tumor entities (Fig. 5). While the prevalence levels described in this study are specific to the reagents and the protocol used in our laboratory, it should be expected that the standardized use of other specific antibodies should result in a similar ranking order. In an earlier study, 2,500 cancers from 60 different tumor entities had been analyzed in a study applying a different GATA3 antibody, another protocol, and other scoring criteria [33]. Although these authors found markedly higher GATA3 positivity rates in most tumor types (indicated in Fig. 5) than in our study, the ranking order was indeed highly comparable to the data obtained in our study.
Fig. 5.
GATA3 positivity in the literature. An “X” indicates the fraction of GATA3-positive tumors in the present study, and dots indicate the reported frequencies from the literature for comparison: red dots mark studies with ≤10 tumors, yellow dots mark studies with 11–25 tumors, and black dots mark studies with >25 tumors. The blue rhombus highlights results from the large multi-tumor study by Miettinen et al. [33].
In summary, our data demonstrate that detectable GATA3 expression can occur in various tumor entities including breast, urothelial, salivary gland, squamous cell, and other tumors. Particularly, high frequency and levels of GATA3 occur in breast and urothelial carcinoma. A reduced level of GATA3 reflects cancer progression and poor patient prognosis in breast cancer.
Statement of Ethics
Consent was not required due to local/national guidelines. The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12) and by the Local Ethics Committee (Ethics Commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Conflict of Interest Statement
The monoclonal mouse GATA3 antibody, clone MSVA-450M, was provided from MS Validated Antibodies GmbH (owned by a family member of GS).
Funding Sources
No funding was received.
Author Contributions
Viktor Reiswich, Ronald Simon, Guido Sauter, and Frank Jacobsen contributed to conception, design, data collection, data analysis, and manuscript writing. Carol E. Schmidt, Sören Weidemann, Franzisak Büscheck, Claudia Hube-Magg, Doris Höflmayer, Christian Bernreuther, Stefan Steurer, and Sarah Minner participated in pathology data analysis and data interpretation. Katharina Möller, Ria Uhlig, Christoph Fraune, Maximilian Lennartz, Sören Weidemann, Andreas H. Marx, Franziska Büscheck, Doris Höflmayer, Christian Bernreuther, Patrick Lebok, Guido Sauter, Stefan Steurer, Eike Burandt, David Dum, Till Krech, Sarah Minner, Frank Jacobsen, Till S. Clauditz, and Andrea Hinsch: collection of samples. Ronald Simon, Niclas C. Blessin, Elana Bady, Claudia Hube-Magg, Viktor Reiswich, and Frank Jacobsen: data analysis. Viktor Reiswich, Frank Jacobsen, and Guido Sauter: study supervision. All authors agreed to be accountable for the content of the work.
Data Availability Statement
Further inquiries can be directed to the corresponding author. Raw data are available upon reasonable request. All data relevant to the study are included in the article and its online supplementary material.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
We are grateful to Melanie Witt, Inge Brandt, Maren Eisenberg, Laura Behm, and Sünje Seekamp for excellent technical assistance.
Funding Statement
No funding was received.
References
- 1.Labastie MC, Catala M, Gregoire JM, Peault B. The GATA-3 gene is expressed during human kidney embryogenesis. Kidney Int. 1995;47((6)):1597–15603. doi: 10.1038/ki.1995.223. [DOI] [PubMed] [Google Scholar]
- 2.Pei XH, Bai F, Smith MD, Usary J, Fan C, Pai SY, et al. CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell. 2009;15((5)):389–401. doi: 10.1016/j.ccr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13((2)):141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishwick C, Higgins J, Percival-Alwyn L, Hustler A, Pearson J, Bastkowski S, et al. Heterarchy of transcription factors driving basal and luminal cell phenotypes in human urothelium. Cell Death Differ. 2017;24((5)):809–818. doi: 10.1038/cdd.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133((1)):53–61. doi: 10.1242/dev.02184. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JPT, Kaygusuz G, Wang L, Montgomery K, Mason V, Zhu SX, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31((5)):673–680. doi: 10.1097/01.pas.0000213438.01278.5f. [DOI] [PubMed] [Google Scholar]
- 7.Krendl C, Shaposhnikov D, Rishko V, Ori C, Ziegenhain C, Sass S, et al. GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc Natl Acad Sci U S A. 2017;114((45)):E9579–88. doi: 10.1073/pnas.1708341114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirkovic J, Elias K, Drapkin R, Barletta JA, Quade B, Hirsch MS. GATA3 expression in gestational trophoblastic tissues and tumours. Histopathology. 2015;67((5)):636–644. doi: 10.1111/his.12681. [DOI] [PubMed] [Google Scholar]
- 9.Wan YY. GATA3: a master of many trades in immune regulation. Trends Immunol. 2014;35((6)):233–242. doi: 10.1016/j.it.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takemoto N, Arai KI, Miyatake S. Cutting edge: the differential involvement of the N-finger of GATA-3 in chromatin remodeling and transactivation during Th2 development. J Immunol. 2002;169((8)):4103–417. doi: 10.4049/jimmunol.169.8.4103. [DOI] [PubMed] [Google Scholar]
- 11.Zhang DH, Yang L, Cohn L, Parkyn L, Homer R, Ray P, et al. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11((4)):473–482. doi: 10.1016/s1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
- 12.El-Assaad A, Dawy Z, Khalil A, Nemer G. Mutational signatures in GATA3 transcription factor and its DNA binding domain that stimulate breast cancer and HDR syndrome. Sci Rep. 2021;11((1)):22762. doi: 10.1038/s41598-021-01832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takaku M, Grimm SA, De Kumar B, Bennett BD, Wade PA. Cancer-specific mutation of GATA3 disrupts the transcriptional regulatory network governed by Estrogen Receptor alpha, FOXA1 and GATA3. Nucleic Acids Res. 2020;48((9)):4756–4768. doi: 10.1093/nar/gkaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanga S, Broom BM, Cristini V, Edgerton ME. Gene expression meta-analysis supports existence of molecular apocrine breast cancer with a role for androgen receptor and implies interactions with ErbB family. BMC Med Genomics. 2009;2((1)):59. doi: 10.1186/1755-8794-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson BJ, Giguere V. Meta-analysis of human cancer microarrays reveals GATA3 is integral to the estrogen receptor alpha pathway. Mol Cancer. 2008;7((1)):49. doi: 10.1186/1476-4598-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J. GATA3 regulates the development and functions of innate lymphoid cell subsets at multiple stages. Front Immunol. 2017;8:1571. doi: 10.3389/fimmu.2017.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantilla JG, Antic T, Tretiakova M. GATA3 as a valuable marker to distinguish clear cell papillary renal cell carcinomas from morphologic mimics. Hum Pathol. 2017;66:152–158. doi: 10.1016/j.humpath.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Takada N, Hirokawa M, Suzuki A, Higuchi M, Kuma S, Miyauchi A. Diagnostic value of GATA-3 in cytological identification of parathyroid tissues. Endocr J. 2016;63((7)):621–626. doi: 10.1507/endocrj.EJ15-0700. [DOI] [PubMed] [Google Scholar]
- 19.Mertens RB, de Peralta-Venturina MN, Balzer BL, Frishberg DP. GATA3 expression in normal skin and in benign and malignant epidermal and cutaneous adnexal neoplasms. Am J Dermatopathol. 2015;37((12)):885–891. doi: 10.1097/DAD.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banet N, Gown AM, Shih IM, Kay Li Q, Roden RB, Nucci MR, et al. GATA-3 expression in trophoblastic tissues: an immunohistochemical study of 445 cases, including diagnostic utility. Am J Surg Pathol. 2015;39((1)):101–108. doi: 10.1097/PAS.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ordonez NG. Value of GATA3 immunostaining in the diagnosis of parathyroid tumors. Appl Immunohistochem Mol Morphol. 2014;22((10)):756–761. doi: 10.1097/PAI.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LE, Begum S, Westra WH, Bishop JA. GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol. 2013;7((4)):311–315. doi: 10.1007/s12105-013-0442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Shi J, Wilkerson ML, Lin F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138((1)):57–64. doi: 10.1309/AJCP5UAFMSA9ZQBZ. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Antic T, Witten D, Paner GP, Taxy JB, Husain A, et al. Is GATA3 expression maintained in regional metastases?: a study of paired primary and metastatic urothelial carcinomas. Am J Surg Pathol. 2013;37((12)):1876–1881. doi: 10.1097/PAS.0b013e31829e2525. [DOI] [PubMed] [Google Scholar]
- 25.Stolnicu S, Tunde C, Cadar A, Boros M. Differences in GATA3 expression among histological/molecular subtypes and grades in infiltrating breast carcinoma (IBC) are important in the diagnosis of metastatic breast carcinoma. Pol J Pathol. 2020;71((1)):62–65. doi: 10.5114/pjp.2020.95417. [DOI] [PubMed] [Google Scholar]
- 26.Kutasovic JR, McCart Reed AE, Males R, Sim S, Saunus JM, Dalley A, et al. Breast cancer metastasis to gynaecological organs: a clinico-pathological and molecular profiling study. J Pathol Clin Res. 2019;5((1)):25–39. doi: 10.1002/cjp2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol. 2010;23((5)):654–661. doi: 10.1038/modpathol.2010.38. [DOI] [PubMed] [Google Scholar]
- 28.Ciocca V, Daskalakis C, Ciocca RM, Ruiz-Orrico A, Palazzo JP. The significance of GATA3 expression in breast cancer: a 10-year follow-up study. Hum Pathol. 2009;40((4)):489–495. doi: 10.1016/j.humpath.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Cakir A, Isik Gonul I, Ekinci O, Cetin B, Benekli M, Uluoglu O. GATA3 expression and its relationship with clinicopathological parameters in invasive breast carcinomas. Pathol Res Pract. 2017;213((3)):227–234. doi: 10.1016/j.prp.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Laurent E, Begueret H, Bonhomme B, Veillon R, Thumerel M, Velasco V, et al. SOX10, GATA3, GCDFP15, androgen receptor, and mammaglobin for the differential diagnosis between triple-negative breast cancer and TTF1-negative lung adenocarcinoma. Am J Surg Pathol. 2019;43((3)):293–302. doi: 10.1097/PAS.0000000000001216. [DOI] [PubMed] [Google Scholar]
- 31.Voduc D, Cheang M, Nielsen T. GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer Epidemiol Biomarkers Prev. 2008;17((2)):365–373. doi: 10.1158/1055-9965.EPI-06-1090. [DOI] [PubMed] [Google Scholar]
- 32.Dang DN, Raj G, Sarode V, Molberg KH, Vadlamudi RK, Peng Y. Significantly increased PELP1 protein expression in primary and metastatic triple-negative breast carcinoma: comparison with GATA3 expression and PELP1's potential role in triple-negative breast carcinoma. Hum Pathol. 2015;46((12)):1829–1835. doi: 10.1016/j.humpath.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38((1)):13–22. doi: 10.1097/PAS.0b013e3182a0218f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jangir H, Nambirajan A, Seth A, Sahoo RK, Dinda AK, Nayak B, et al. Prognostic stratification of muscle invasive urothelial carcinomas using limited immunohistochemical panel of Gata3 and cytokeratins 5/6, 14 and 20. Ann Diagn Pathol. 2019;43:151397. doi: 10.1016/j.anndiagpath.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Leivo MZ, Elson PJ, Tacha DE, Delahunt B, Hansel DE. A combination of p40, GATA-3 and uroplakin II shows utility in the diagnosis and prognosis of muscle-invasive urothelial carcinoma. Pathology. 2016;48((6)):543–549. doi: 10.1016/j.pathol.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Mai KT, Bateman J, Djordjevic B, Flood TA, Belanger EC. Clear cell urothelial carcinoma. Int J Surg Pathol. 2017;25((1)):18–25. doi: 10.1177/1066896916660195. [DOI] [PubMed] [Google Scholar]
- 37.Agaimy A, Bertz S, Cheng L, Hes O, Junker K, Keck B, et al. Loss of expression of the SWI/SNF complex is a frequent event in undifferentiated/dedifferentiated urothelial carcinoma of the urinary tract. Virchows Arch. 2016;469((3)):321–330. doi: 10.1007/s00428-016-1977-y. [DOI] [PubMed] [Google Scholar]
- 38.Wang CC, Tsai YC, Jeng YM. Biological significance of GATA3, cytokeratin 20, cytokeratin 5/6 and p53 expression in muscle-invasive bladder cancer. PLoS One. 2019;14((8)):e0221785. doi: 10.1371/journal.pone.0221785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyamoto H, Izumi K, Yao JL, Li Y, Yang Q, McMahon LA, et al. GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum Pathol. 2012;43((11)):2033–2040. doi: 10.1016/j.humpath.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Mohammed KH, Siddiqui MT, Cohen C. GATA3 immunohistochemical expression in invasive urothelial carcinoma. Urol Oncol. 2016;34((10)):432 e9–13. doi: 10.1016/j.urolonc.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Hoang LL, Tacha D, Bremer RE, Haas TS, Cheng L. Uroplakin II (UPII), GATA3, and p40 are highly sensitive markers for the differential diagnosis of invasive urothelial carcinoma. Appl Immunohistochem Mol Morphol. 2015;23((10)):711–716. doi: 10.1097/PAI.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 42.Tian W, Guner G, Miyamoto H, Cimino-Mathews A, Gonzalez-Roibon N, Argani P, et al. Utility of uroplakin II expression as a marker of urothelial carcinoma. Hum Pathol. 2015;46((1)):58–64. doi: 10.1016/j.humpath.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Roibon N, Albadine R, Sharma R, Faraj SF, Illei PB, Argani P, et al. The role of GATA binding protein 3 in the differential diagnosis of collecting duct and upper tract urothelial carcinomas. Hum Pathol. 2013;44((12)):2651–267. doi: 10.1016/j.humpath.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Ellis CL, Chang AG, Cimino-Mathews A, Argani P, Youssef RF, Kapur P, et al. GATA-3 immunohistochemistry in the differential diagnosis of adenocarcinoma of the urinary bladder. Am J Surg Pathol. 2013;37((11)):1756–1760. doi: 10.1097/PAS.0b013e31829cdba7. [DOI] [PubMed] [Google Scholar]
- 45.Kandalaft PL, Simon RA, Isacson C, Gown AM. Comparative sensitivities and specificities of antibodies to breast markers GCDFP-15, mammaglobin A, and different clones of antibodies to GATA-3: a study of 338 tumors using whole sections. Appl Immunohistochem Mol Morphol. 2016;24((9)):609–614. doi: 10.1097/PAI.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 46.Hui Y, Wang Y, Nam G, Fanion J, Sturtevant A, Lombardo KA, et al. Differentiating breast carcinoma with signet ring features from gastrointestinal signet ring carcinoma: assessment of immunohistochemical markers. Hum Pathol. 2018;77:11–19. doi: 10.1016/j.humpath.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keshari RP, Wang W, Zhang Y, Wang DD, Li YF, Yuan SQ, et al. Decreased expression of the GATA3 gene is associated with poor prognosis in primary gastric adenocarcinoma. PLoS One. 2014;9((2)):e87195. doi: 10.1371/journal.pone.0087195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nhung NV, Mirejovsky P, Mirejovsky T, Melinova L. Cytokeratins and lung carcinomas. Cesk Patol. 1999;35((3)):80–84. [PubMed] [Google Scholar]
- 49.Chen Y, Cui T, Yang L, Mireskandari M, Knoesel T, Zhang Q, et al. The diagnostic value of cytokeratin 5/6, 14, 17, and 18 expression in human non-small cell lung cancer. Oncology. 2011;80((5–6)):333–340. doi: 10.1159/000329098. [DOI] [PubMed] [Google Scholar]
- 50.Hashiguchi T, Miyoshi H, Nakashima K, Yokoyama S, Matsumoto R, Murakami D, et al. Prognostic impact of GATA binding protein-3 expression in primary lung adenocarcinoma. Hum Pathol. 2017;63:157–164. doi: 10.1016/j.humpath.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 51.Brunelli M, Erdini F, Cima L, Eccher A, Fioravanzo A, Gobbo S, et al. Proximal CD13 versus distal GATA-3 expression in renal neoplasia according to WHO 2016 classification. Appl Immunohistochem Mol Morphol. 2018;26((5)):316–323. doi: 10.1097/PAI.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 52.Betts G, Beckett E, Nonaka D. GATA3 shows differential immunohistochemical expression across thyroid and parathyroid lesions. Histopathology. 2014;65((2)):288–290. doi: 10.1111/his.12388. [DOI] [PubMed] [Google Scholar]
- 53.Terzic T, Mills AM, Zadeh S, Atkins KA, Hanley KZ. GATA3 expression in common gynecologic carcinomas: a potential pitfall. Int J Gynecol Pathol. 2019;38((5)):485–492. doi: 10.1097/PGP.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 54.Engelsen IB, Stefansson IM, Akslen LA, Salvesen HB. GATA3 expression in estrogen receptor alpha-negative endometrial carcinomas identifies aggressive tumors with high proliferation and poor patient survival. Am J Obstet Gynecol. 2008;199((5)):543 e1–7. doi: 10.1016/j.ajog.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 55.Dancau AM, Simon R, Mirlacher M, Sauter G. Tissue Microarrays. Methods Mol Biol. 2016;1381:53–65. doi: 10.1007/978-1-4939-3204-7_3. [DOI] [PubMed] [Google Scholar]
- 56.Kononen J, Bubendorf L, Kallionimeni A, Barlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4((7)):844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 57.Simon R, Mirlacher M, Sauter G. Immunohistochemical analysis of tissue microarrays. Methods Mol Biol. 2010;664:113–126. doi: 10.1007/978-1-60761-806-5_12. [DOI] [PubMed] [Google Scholar]
- 58.Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, et al. A proposal for validation of antibodies. Nat Methods. 2016;13((10)):823–827. doi: 10.1038/nmeth.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Human Protein Atlas ALPP, RNA Database, version. 20.1.proteinatlas.org [Internet]. Available from: https://www.proteinatlas.org/ENSG00000163283-ALPP/tissue.
- 60.Lizio M, Abugessaisa I, Noguchi S, Kondo A, Hasegawa A, Hon CC, et al. Update of the FANTOM web resource: expansion to provide additional transcriptome atlases. Nucleic Acids Res. 2019;47((D1)):D752–8. doi: 10.1093/nar/gky1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lizio M, Harshbarger J, Shimoji H, Severin J, Kasukawa T, Sahin S, et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015;16((1)):22. doi: 10.1186/s13059-014-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45((6)):580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. 2009;9((2)):125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang HL, Kim CJ, Koo J, Zhou W, Choi EK, Arcega R, et al. Practical immunohistochemistry in neoplastic pathology of the gastrointestinal tract, liver, biliary tract, and pancreas. Arch Pathol Lab Med. 2017;141((9)):1155–1180. doi: 10.5858/arpa.2016-0489-RA. [DOI] [PubMed] [Google Scholar]
- 65.Lin F, Liu H. Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. Arch Pathol Lab Med. 2014;138((12)):1583–15610. doi: 10.5858/arpa.2014-0061-RA. [DOI] [PubMed] [Google Scholar]
- 66.Tjarks BJ, Pownell BR, Evans C, Thompson PA, Kerkvliet AM, Koch MRD, et al. Evaluation and comparison of staining patterns of factor XIIIa (AC-1A1), adipophilin and GATA3 in sebaceous neoplasia. J Cutan Pathol. 2018;45((1)):1–7. doi: 10.1111/cup.13037. [DOI] [PubMed] [Google Scholar]
- 67.Pardal J, Sundram U, Selim MA, Hoang MP. GATA3 and MYB expression in cutaneous adnexal neoplasms. Am J Dermatopathol. 2017;39((4)):279–286. doi: 10.1097/DAD.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 68.Edqvist PHD, Fagerberg L, Hallstrom BM, Danielsson A, Edlund K, Uhlen M, et al. Expression of human skin-specific genes defined by transcriptomics and antibody-based profiling. J Histochem Cytochem. 2015;63((2)):129–141. doi: 10.1369/0022155414562646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cairns P. Renal cell carcinoma. Cancer Biomark. 2011;9((1–6)):461–473. doi: 10.3233/CBM-2011-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xin L. Cells of origin for prostate cancer. Adv Exp Med Biol. 2019;1210:67–86. doi: 10.1007/978-3-030-32656-2_4. [DOI] [PubMed] [Google Scholar]
- 71.Babu S, Agarwal H, Rana C, Kumar M, Singhai A, Shankhwar SN, et al. Diagnostic utility of GATA3 immunohistochemical expression in urothelial carcinoma. Indian J Pathol Microbiol. 2019;62((2)):244–250. doi: 10.4103/IJPM.IJPM_228_18. [DOI] [PubMed] [Google Scholar]
- 72.Siddiqui MT, Seydafkan S, Cohen C. GATA3 expression in metastatic urothelial carcinoma in fine needle aspiration cell blocks: a review of 25 cases. Diagn Cytopathol. 2014;42((9)):809–815. doi: 10.1002/dc.23131. [DOI] [PubMed] [Google Scholar]
- 73.Bejrananda T, Kanjanapradit K, Saetang J, Sangkhathat S. Impact of immunohistochemistry-based subtyping of GATA3, CK20, CK5/6, and CK14 expression on survival after radical cystectomy for muscle-invasive bladder cancer. Sci Rep. 2021;11((1)):21186. doi: 10.1038/s41598-021-00628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian W, Dorn D, Wei S, Sanders RD, Matoso A, Shah RB, et al. GATA3 expression in benign prostate glands with radiation atypia: a diagnostic pitfall. Histopathology. 2017;71((1)):150–155. doi: 10.1111/his.13214. [DOI] [PubMed] [Google Scholar]
- 75.Shield PW, Crouch SJ, Papadimos DJ, Walsh MD. Gata3 immunohistochemical staining is A useful marker for metastatic breast carcinoma in fine needle aspiration specimens. J Cytol. 2018;35((2)):90–93. doi: 10.4103/JOC.JOC_132_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wendroth SM, Mentrikoski MJ, Wick MR. GATA3 expression in morphologic subtypes of breast carcinoma: a comparison with gross cystic disease fluid protein 15 and mammaglobin. Ann Diagn Pathol. 2015;19((1)):6–9. doi: 10.1016/j.anndiagpath.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Albergaria A, Paredes J, Sousa B, Milanezi F, Carneiro V, Bastos J, et al. Expression of FOXA1 and GATA-3 in breast cancer: the prognostic significance in hormone receptor-negative tumours. Breast Cancer Res. 2009;11((3)):R40. doi: 10.1186/bcr2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Querzoli P, Pedriali M, Rinaldi R, Secchiero P, Rossi PG, Kuhn E. GATA3 as an adjunct prognostic factor in breast cancer patients with less aggressive disease: a study with a review of the literature. Diagnostics. 2021;11((4)):604. doi: 10.3390/diagnostics11040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yildirim E, Bektas S, Gundogar O, Findik D, Alcicek S, Erdogan KO, et al. The relationship of GATA3 and ki-67 with histopathological prognostic parameters, locoregional recurrence and disease-free survival in invasive ductal carcinoma of the breast. Anticancer Res. 2020;40((10)):5649–5657. doi: 10.21873/anticanres.14578. [DOI] [PubMed] [Google Scholar]
- 80.Tominaga N, Naoi Y, Shimazu K, Nakayama T, Maruyama N, Shimomura A, et al. Clinicopathological analysis of GATA3-positive breast cancers with special reference to response to neoadjuvant chemotherapy. Ann Oncol. 2012;23((12)):3051–307. doi: 10.1093/annonc/mds120. [DOI] [PubMed] [Google Scholar]
- 81.Liu J, Prager-van der Smissen WJ, Look MP, Sieuwerts AM, Smid M, Meijer-van Gelder ME, et al. GATA3 mRNA expression, but not mutation, associates with longer progression-free survival in ER-positive breast cancer patients treated with first-line tamoxifen for recurrent disease. Cancer Lett. 2016;376((1)):104–109. doi: 10.1016/j.canlet.2016.03.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
Further inquiries can be directed to the corresponding author. Raw data are available upon reasonable request. All data relevant to the study are included in the article and its online supplementary material.