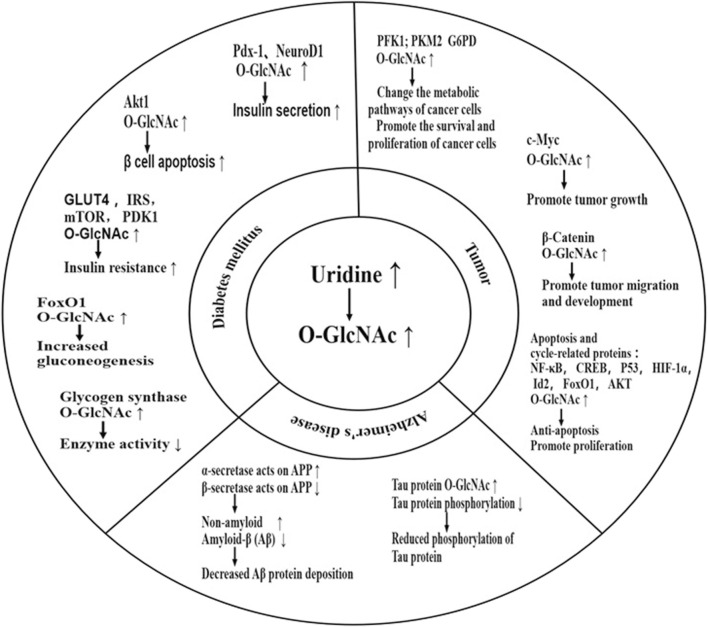
FIGURE 3.
Effect of uridine on disease through O-GlcNAcylation. Uridine can impact diseases by increasing O-GlcNAc of important functional proteins. Current research focuses mainly on diabetes, Alzheimer’s disease, and cancer. Elevated O-GlcNAc levels of specific proteins can worsen blood glucose status by promoting βcell apoptosis, exacerbating insulin resistance, inhibiting glycogen synthesis, promoting gluconeogenesis, among other mechanisms. However, in the short term, it can also promote insulin secretion, partially explaining the differences in the effects of urinary nucleosides on diabetic patients in the short and long term. The typical pathological manifestation of Alzheimer’s disease is hyperphosphorylation of Tau protein and deposition of β-amyloid protein. Supplementation with uridine can provide neuroprotection by increasing O-GlcNAc of Tau protein, reducing Tau phosphorylation, and preventing Tau aggregation. It can also shift the processing of amyloid precursor protein (APP) towards the non-amyloidogenic pathway mediated by α-secretase and away from the amyloidogenic pathway mediated by β-secretase, reducing the production and deposition of β-amyloid protein-induced neurotoxicity. However, high levels of O-GlcNAc can promote tumor development by promoting tumor cell proliferation, inhibiting tumor cell apoptosis, and promoting tumor cell migration. PDX-1:pancreatic and duodenal homeobox-1; NeuroD1:neurogenic differentiation 1; AKT: kinase B; G6PD: Glucose-6-phosphate dehydrogenase; PDK1: phosphoinositide-dependent kinase 1; PFK1: Phosphofructokinase-1; PKM2: pyruvate kinase M2 isoform; IRS: insulin receptor substrate; APP: Amyloid precursor protein; FoxO1:forkhead box O1; HIF-1α:hypoxia-inducible factor 1 α; CREB:cAMP response element-binding protein; Id2:inhibitor of differentiation 2.