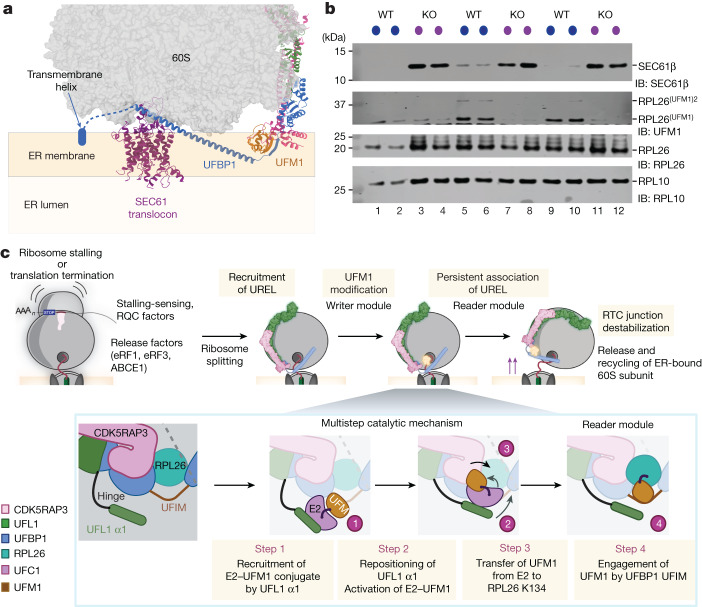
Fig. 5. UFMylation of RPL26 mediates dissociation of the 60S ribosomal subunit from the ER translocon.
a, UREL–UFM1 superimposed onto the structure of 80S–SEC61 (PDB: 6R7Q). Modelling UREL binding to SEC61 translocon-bound ribosomes suggests that the ligase complex will destabilize the 60S–SEC61 interaction. The approximate position of the ER membrane and ER lumen relative to the SEC61 translocon are shown. b, Loss of UFMylation leads to an accumulation of 60S ribosomes with the translocon. SEC61β association with 60S ribosomes was analysed in membrane fractions from parental WT and CDK5RAP3-KO cells. Lysates were normalized to uniform RNA concentration (using absorbance at 254 nm) and fractionated on 10–30% sucrose density gradients. The fractions corresponding to 60S from three independent replicates were analysed by immunoblotting using the indicated antibodies. c, Model for 60S–SEC61 translocon recognition by UREL, showing the multistep catalytic mechanism leading to RPL26 UFMylation and the subsequent conversion of UREL to a reader that splits the 60S ribosomal subunit from the translocon (see Discussion). The diagram in c was created using BioRender.