Figure 9.
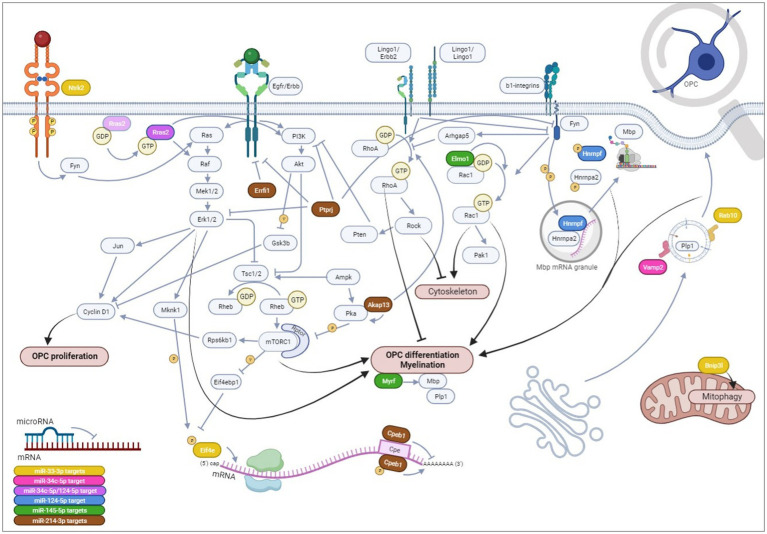
Hypotheses on the mechanistical/functional involvement of each microRNA and its potential mRNA targets on OPC differentiation. We have evidenced that miR-33-3p, miR-34c-5p, miR-124-5p, and miR-145-5p impede OPC differentiation, while miR-214-3p promotes it in CG-4 cells, an OPC cell line. Herein, we hypothesize that miR-33-3p (yellow) targets: (1) Eif4e, a translation initiation factor, part of the Eif4f complex, that once phosphorylated recognizes the mRNA 5′-terminal 7-methylguanosine (m7G) cap on OPC differentiation and myelin genes downstream PI3K/mTOR and ERK/MAPK signaling; (2) Rab10, a small GTPase involved in intracellular vesicle trafficking allowing plasma membrane elongation important for myelin ensheathment; (3) Bnip3l regulating mitochondrial function by mitophagy; and (4) more hypothetically Ntrk2 upstream of the Ras/ERK/MAPK cascade. miR-34c-5p possibly targets Vamp2 (pink), involved in intracellular vesicle targeting to the plasma membrane, leading to its expansion, and potentially Rras2 (purple), a small GTPase involved in OPC differentiation by inducing ERK/MAPK and PI3K/Akt/mTOR signaling pathways. Rras2 is also a predicted target of miR-124-5p. miR-124-5p is further predicted to target Hnrnpf (blue) that, upon phosphorylation by Fyn tyrosine kinase within Mbp transport granules, releases Mbp mRNA, allowing its translation within the cellular processes and its local interaction with the cytoplasmic membrane leaflets inducing myelin compaction. miR-145-5p (green) has already been proven to target Myrf, a key transcription factor of myelin genes, and possibly targets Elmo1 as well, an activator of Rac1, a small GTPase (which is itself induced by Fyn tyrosine kinase by integrin engagement) inducing morphological OPC differentiation by acting on actin organization, or possibly via p21 activated kinase 1 (Pak1). Finally, miR-214-3p (brown), the only microRNA of our study inducing OPC differentiation, hypothetically targets (1) Akap13, a scaffolder of protein kinase A (Pka) that inhibits mTORC1 and an inducer of RhoA (which is itself inhibited by Fyn but induced by Lingo1) that was found to inhibit OPC differentiation by favoring stress fiber formation and actin depolymerization, or potentially by inducing Pten, an inhibitor of PI3K/Akt/mTOR signaling; (2) Cpeb1, acting as a translational repressor until it is phosphorylated or degraded, with its consensus binding sequence on several OPC/OL-related genes; (3) Errfi1, an Erbb receptor feedback inhibitor, and (4) Ptprj, a protein tyrosine phosphatase, both inhibiting Erbb signaling that can be involved in OPC differentiation. Ptprj can dephosphorylate ERK/MAPK, PI3K, and Fyn tyrosine kinase as well. Notably, the Erbb receptor tyrosine kinase family, existing of 4 isoforms, is important in OPC/OL physiology. Upon dimerization of two receptors within the Erbb family, which will determine the downstream signaling of the receptor tyrosine kinase, the signal will mainly be transduced intracellularly to the PI3K/Akt/mTOR signaling pathway, inducing OPC differentiation and myelination, or to the ERK/MAPK cascade, enhancing myelin thickness, although both have also been described as playing a role in OPC proliferation upon Pdgf induction through cyclin D1 (Ccnd1). OPC,oligodendrocyte progenitor cells, OL, oligodendrocytes. All references supporting these hypotheses are cited within the article, except Rac1/Pak1 and Lingo1/Erbb2 interactions (Parrini et al., 2002; Mi et al., 2013; Brown et al., 2021). Created in BioRender.com.