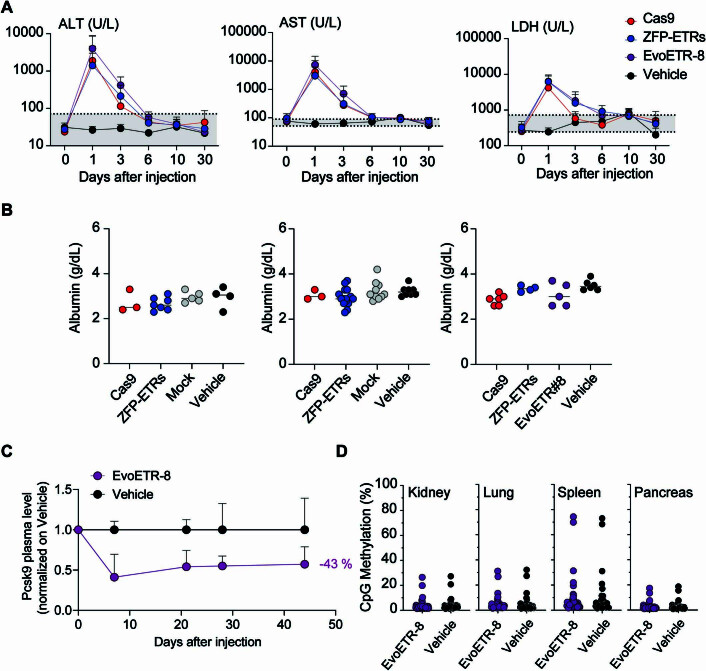
Extended Data Fig. 5. Efficacy, biodistribution and liver toxicity of LNP-mediated delivery of ETRs.
a, Time-course analysis of plasma levels of ALT, AST and LDH after LNP-mediated delivery of mRNAs encoding for either Cas9, ZFP-ETRs, or EvoETR-8. Data are reported as mean ± s.d. (n = 6). b, Quantification of circulating albumin at the last time point analysed. Data are reported as individual values (dots) and medians (lines). Left: experiment in Fig. 3b, 330 days post-injection; centre: experiment in Extended Data Fig. 3g, 70 days post-injection; right: experiment in Fig. 5b, 43 days post-injection. c, Time course of circulating Pcsk9 until day 44 post-injection of GenVoy-LNPs encapsulating the mRNA of EvoETR-8. Data are reported as mean ± s.d. and normalized to the PCSK9 levels of vehicle-treated mice (n = 5 for EvoETR-8- and 2 for vehicle-injected mice). d, Dot plots showing the percentage of CpG methylation at the Pcsk9 promoter in the indicated organs from EvoETR-8- and vehicle-treated mice. Each dot represents a single CpG (mean of n = 3).