Figure 1. The novel PTGS1 variant p.Asn143Ser affecting the N-glycosylation residue 143 in COX-1, results in aspirin- like impaired platelet aggregation and expression of a hypo-glycosylated and dysfunctional COX-1 enzyme in human platelet and cell models.
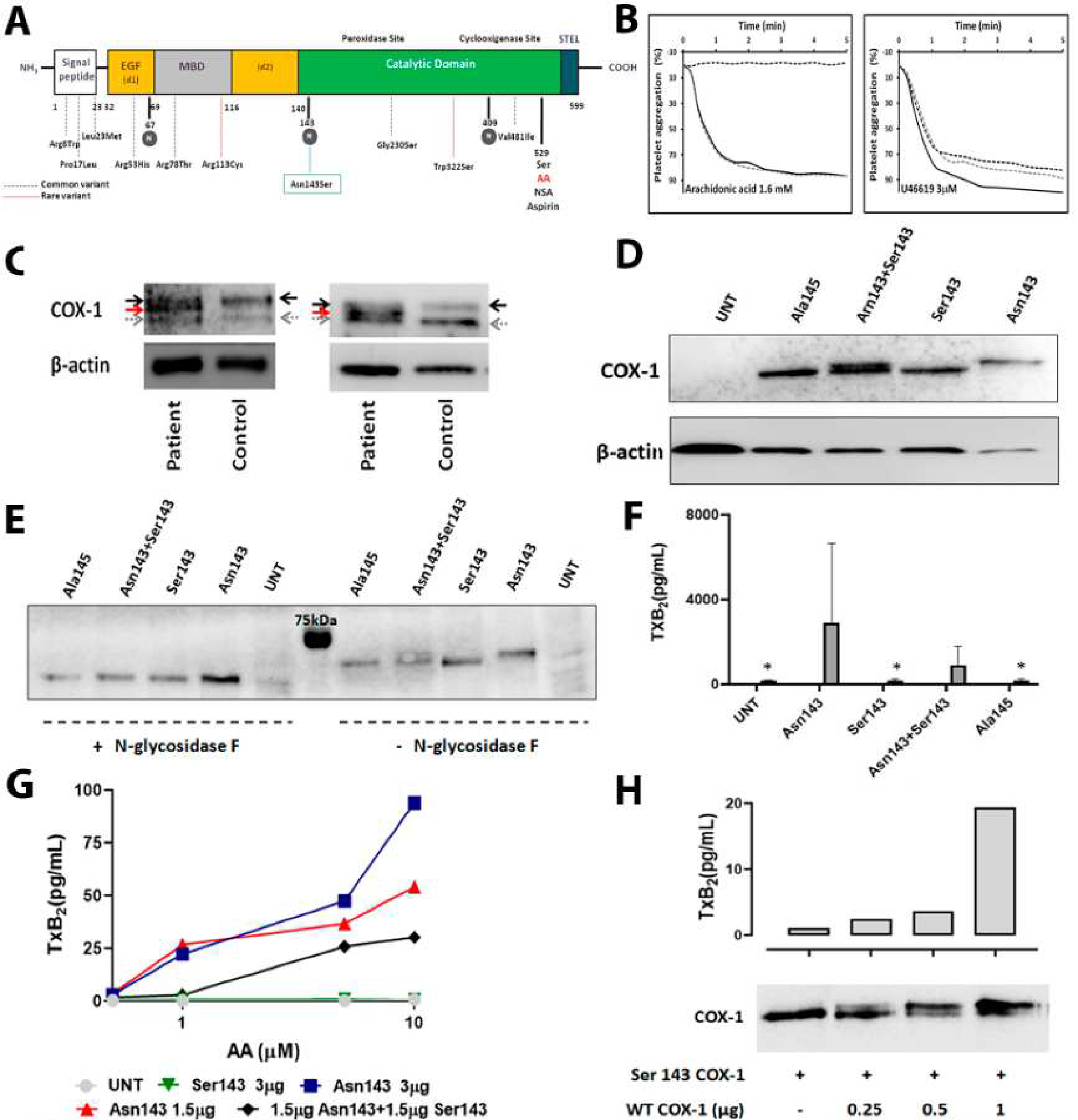
A) Schematic representation for COX-1 showing the different domains and the localization of the novel p.Asn143Ser genetic variant as well as all previously reported common (dotted blue lines) and rare (dotted red lines) variants. N-glycosylated residues are highlighted in grey circles. B) Platelet aggregation in response to arachidonic acid and U46619, a TxA2 analog, was evaluated in PRP from the proband (black dotted line) and from two healthy controls (grey dotted line and black line). C) Two independent western blots representing COX-1 in platelets from the proband and time-matched control. Platelet lysates were resolved in 8% SDS- polyacrylamide gel for 90 min. In addition to wild-type COX-1 (black arrow), a second COX-1 band with lower molecular weight (red arrow) was detected in the patient. Grey arrow identified a non-specific band. β-actin was used as an internal control. D) Western blot determination of COX-1 expression in 293 T HEK cells untransfected (UNT), transfected with wild-type Asn143 COX-1 vector (Asn143), mutant Ser143 COX-1 vector (Ser143), co-transfected with both vectors (Asn143+Ser143), or transfected with mutant Ala145 COX-1 vector (Ala145), β-actin was used as an internal control. E) Western blots of COX-1 expression in untransfected (UNT) or variant transfected 293 T HEK cells treated (+) or not (−) with N-glycosidase F. F) 293 T HEK cells were untransfected (UNT), transfected with wild-type Asn143 COX-1 vector (Asn143), mutant Ser143 COX-1 vector (Ser143), co-transfected with both vectors (Asn143+Ser143), or transfected with mutant Ala145 COX-1 vector (Ala145). The enzymatic activity of COX-1 was then assessed by using ELISA for TXB2 to assess the formation of TXA2 following incubation with 500 μM arachidonic acid. Plots show mean plus standard deviation from values obtained in three 3 independent experiments. ”*” denotes P value=0.05 vs. Asn143 (U de Mann-Whitney test). G) 293 T HEK cells untransfected (UNT), transfected with different amounts of wild-type Asn143 COX-1 vector, transfected with mutant Ser143 COX-1 vector, or co-transfected with the same amount of both vectors (Asn143+Ser143), were stimulated with different concentrations of AA (0.5 −10 μM) and the formation of TXA2 was assessed by using ELISA. The plot is representative of 2 independent experiments. H) HEK 293 cells were transfected with vector expressing Ser143 mutant COX-1 and the neomicine resistance gen and grown in the presence of geneticine. Resistant and stable transfected cell clones were isolated and then transfected with different amounts of vector (0–1 μg) expressing native Asn143 COX-1. Cells were washed and split for preparation of protein lysates and for arachidonic acid (1 μM) stimulation and TXA2 production measurement by ELISA. A double COX-1 protein is seen in co-transfected cells. Native COX-1 recovers TXA2 production, but following a non-linear dose-dependent pattern.
