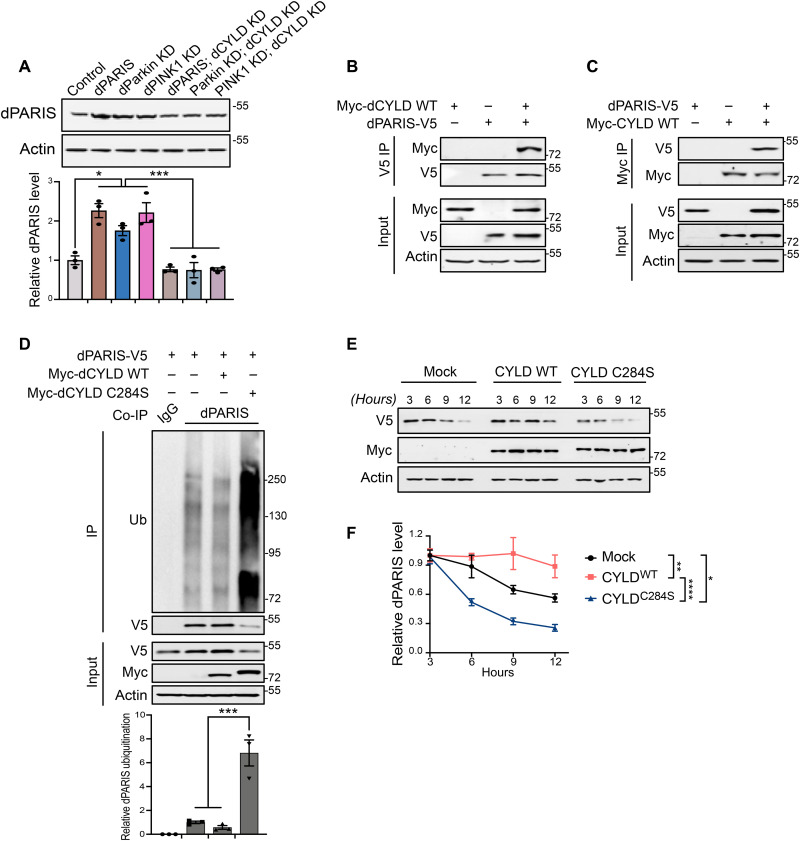
Fig. 4. KD of dCYLD promotes dopamine neuron survival by destabilizing dPARIS through its DUB activity.
(A) Representative immunoblot and dPARIS quantification in flies expressing the indicated transgenes under the control of TH-Gal4 driver. TH-Gal4/+ flies served as control, N = 3. (B) Coimmunoprecipitation using anti-V5 antibodies shows interaction between C-terminal V5-tagged dPARIS and N-terminal Myc-tagged dCYLD in Drosophila S2 cells. Similar results were observed in three independent experiments. (C) Reciprocal coimmunoprecipitation experiments using anti-Myc antibodies verify dPARIS interaction with dCYLD in S2 cells transfected with indicated constructs in three independent experiments, N = 3. (D) Deubiquitination of dPARIS by dCYLD as assessed in S2 cells transfected with indicated constructs. Immunoblot analysis and relative quantification of immunoprecipitated (IP) dPARIS shows increased ubiquitination (Ub) of dPARIS in the presence of the dCYLD C284S catalytic mutant, thereby enhancing its proteasomal degradation, N = 3. (E) DUB activity of dCYLD affects the turnover rate of dPARIS. (F) Immunoblot analysis and dPARIS quantification in S2 cells at the indicated time points shows that while the dCYLD catalytic mutant accelerates dPARIS turnover, overexpression of WT dCYLD increases its half-life, N = 3. Quantitative data = means ± SEM. One-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. See also fig. S4.