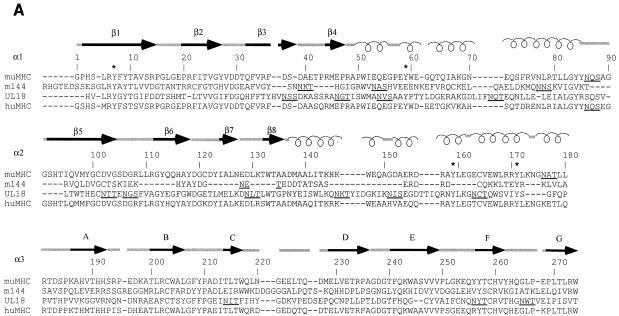
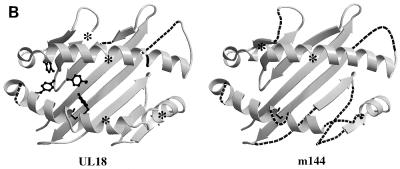
FIG. 1.
Comparison of MCMV and HCMV class I homologs with class I MHC molecules. (A) Sequence alignment of the mature extracellular regions of m144 with a murine class I molecule (muMHC) and of UL18 with a human class I MHC molecule (huMHC) (based on data from Fig. 1 in reference 14). Numbering is with reference to class I MHC molecules. Crystallographically determined secondary-structural elements in class I MHC molecules (3) are shown above the sequences as arrows for β strands (strands 1 through 8 within the α1 and α2 domains are labeled β1 to β8, and strands 1 through 7 within the α3 domain are labeled A to G) and spirals for α-helical regions. Positions of conserved tyrosines in the pocket that accommodates peptide N termini (pocket A in class I MHC molecules [45, 47]) are marked with an asterisk, and potential N-linked glycosylation sites are underlined. (B) Locations of UL18 and m144 sequence insertions and deletions on the class I MHC structure. Ribbon diagrams of the carbon-α backbone of the α1 and α2 domains of HLA-A2 (4, 45) are shown with the locations of UL18 or m144 insertions indicated by asterisks; class I regions that are deleted in UL18 or m144 are indicated by dashed lines. Conserved tyrosines shared between UL18 and class I molecules are highlighted in the left panel. This figure was generated by using Molscript (28) and Raster-3D (34).