Abstract
Background
Red blood cell distribution width (RDW) is calculated in every blood count test and reflects variability in erythrocyte size. High levels mirror dysregulated erythrocyte homeostasis and have been associated with clonal hematopoiesis as well as higher mortality in several conditions.
We aimed to determine the impact of preprocedural RDW levels on functional outcomes after transcatheter aortic valve implantation (TAVI).
Methods
In this single-center retrospective study, we analyzed 176 consecutive patients receiving TAVI between 2017 and 2021. RDW upper limit of normal was < 15 %. Patients were stratified according to preprocedural RDW as having normal or elevated values. We assessed all-cause-mortality and a composite endpoint comprising cardiovascular/ valve-related mortality and cardiovascular, valve-related and heart failure hospitalization at 1 year.
Results
43 patients (24.4 %) had RDW ≥ 15 %. There were significant baseline differences between groups (Society of Thoracic Surgeons – Predicted Risk of Mortality score 3.18 %[interquartile range 1.87–5.47] vs. 6.63 %[4.12–10.54] p < 0.001; hemoglobin 13.2 g/dL[11.8–14.1] vs. 10.4 g/dL[9.8–12.2], p < 0.001, RDW-normal vs. RDW-high, respectively). Age was not distinct (80.2 years [77.5–84.1] vs 81.2[71.3–84.7], p = 0.78). 1-year-all-cause mortality was not different (7.9 % vs. 9.4 %, p = 0.79). The RDW-high group showed markedly higher NT-proBNP levels after 1 year (647 ng/ml[283–1265] vs. 1893 ng/ml[744–5109], p = 0.005), and experienced more clinical endpoints (hazard ratio 2.57[1.28–5.16] for the composite endpoint, p = 0.006). RDW remained an independent predictor of the composite endpoint when accounting for all baseline differences in multivariable regression.
Conclusion
Elevated preprocedural RDW identifies patients at risk for impaired functional outcome after TAVI and may represent a useful low-cost parameter to guide intensity of outpatient surveillance strategies.
Keywords: Aortic stenosis, Transcatheter Aortic Valve Implantation, RDW, CHIP
1. Introduction
Transfemoral transcatheter aortic valve implantation (TAVI) is the treatment of choice for patients with severe aortic stenosis older than 75 years or at high surgical risk and may also be performed in patients with intermediate or low surgical risk [1]. In a relevant proportion of patients, however, TAVI fails to improve heart failure symptoms or overall quality of life and must be considered futile [2]. Although parameters such as preexisting cardiac damage [3], chronic obstructive pulmonary disease [4] or some measures of impaired physical performance [5] may predict futility to some extent, research into predictors of insufficient functional recovery after TAVI is still ongoing.
Red cell distribution (RDW) reflects variability in size of circulating erythrocytes, with high levels mirroring dysregulated erythrocyte homeostasis with either prolonged red blood cell survival or impaired erythropoiesis. While traditionally, RDW is a parameter routinely used in the differential diagnosis of anemia, further relevance for the development of myeloid neoplasm [6], [7] as well as their prognosis [8] became increasingly evident. High levels have also been associated with all-cause death and heart failure hospitalization in patients with chronic heart failure [9], [10] and after acute myocardial infarction [11], and with a composite of all-cause mortality and non-fatal myocardial infarction in patients with chronic coronary syndromes [12]. Elevated RDW at the time of TAVI is associated with increased all-cause mortality in elderly very-high-risk- [13] and high-risk [14]-patients. In intermediate-risk cohorts, RDW might also be elevated, but has, to the best of our knowledge, not been evaluated to date.
Pathophysiologically, elevated RDW may indicate a variety of pathological processes, such as inflammatory stress, hepatic congestion, impaired iron mobilization or undernutrition [10], [15]. While these processes may be difficult to quantitate, RDW is routinely determined in every full blood count.
The value of RDW as a low-cost and readily available parameter to predict functional improvement after transfemoral TAVI has not been evaluated to date. We sought to determine whether elevated RDW also allows the identification of patients at risk for shorter survival, worse functional status, and hospitalizations after TAVI in a contemporary cohort. To the best of our knowledge, this is the first evaluation of the predictive utility of RDW in an intermediate risk cohort.
2. Methods
2.1. Patient cohort
We analyzed consecutive patients receiving transfemoral TAVI for calcific aortic stenosis via our center between June 2017 and December 2022. Baseline characteristics, procedural data and outcome data were retrospectively collected from patient records and telephone interviews. Clinical follow-up evaluations were performed after 1, 6 and 12 months and once a year thereafter. The study was approved by our institutional ethical review board.
We analyzed consecutive patients receiving transfemoral TAVI for calcific aortic stenosis via our center between June 2017 and December 2022. Baseline characteristics, procedural data and outcome data were retrospectively collected from patient records and telephone interviews. Clinical follow-up evaluations were performed after 1, 6 and 12 months and once a year thereafter. The study was approved by our institutional ethical review board.
Presence of comorbidities was defined according to Society of Thoracic Surgeons- Predicted Risk of Mortality (STS-PROM) score, and STS-PROM and EuroSCORE-II were calculated as recommended in current guidelines [1]. Gender was self-reported. Stroke volume index was determined by echocardiography and using the body surface area as determined by the DuBois formula. Glomerular filtration rate was calculated using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) formula.
RDW was routinely determined during full blood count analysis as part of the preprocedural workup within three weeks before valve implantation. RDW is calculated as standard deviation of red blood cell volumes, divided by mean corpuscular volume and then multiplied by 100 to express as a percentage [16]. A RDW of less than 15 % is within normal range at our institution. Hence, patients were stratified as having normal (<15 %) or elevated (≥15 %) RDW.
2.2. Endpoints
Our primary endpoint was a composite of cardiovascular or valve-related death and cardiovascular, valve-related and heart failure-related hospitalization. Cause of death was adjudicated as valve-related, cardiovascular or non-cardiovascular and hospitalization was determined to be valve-related, heart failure related, of other cardiovascular origin or non-cardiovascular according to Valve Academic Research Consortium −3 (VARC-3) [17] with heart failure related hospitalization reported as separate category. Periprocedural events and composite endpoints of ‘technical success’ and ‘early safety’ were also adjudicated according to VARC-3.
2.3. Statistical analysis
Statistical analysis was performed using SPSS Statistics version 22.0 (IBM Corporation, Armonk, NY, USA). Characteristics of both groups are reported as absolute and relative frequencies for categorical variables and median and interquartile range for ordinal and continuous variables. Denominator of proportions may differ because of missing values which were not imputed. Categorical variables were compared by χ2-test. Continuous variables were assessed for distribution normality by Shapiro-Wilk test, and as normal distribution was not present, compared by Mann-Whitney test. Comparison between NT-proBNP values before TAVI and at 1 month and 12 months after TAVI were performed by Wilcoxon’s signed rank test. Survival and incidence of composite endpoints were plotted by Kaplan-Meier method and compared by Log-Rank test. To prevent bias in this retrospective analysis, we built multiple Cox regression models, starting with baseline traits which were significantly different between patients with RDW < 15 % and patients with RDW ≥ 15 %. Variables reaching p < 0.1 were entered into a multivariate Cox regression model. Age was forced into the model. Backward selection by change of likelihood ratio after removal of the respective variable was used as criterion for the variable to remain in the model. We defined significance level at 5 % for two-tailed testing.
3. Results
Between June 2017 and December 2021, 176 patients (38.1 % female, median age 80.4 years, Interquartile range [IQR] 77.0 – 84.5) received transfemoral TAVI. All had baseline RDW levels available. Median preprocedural RDW was 13.9 % (IQR 13.1–14.9), and 24.4 % of patients (n = 43) had RDW of 15 % or above (RDW-high). Median follow-up was 688 days (IQR 390 – 1094 days). Follow-up at 1 year was available for 88.1 % of patients (n = 155; RDW-normal: n = 117 [88.0 %]; RDW-high: n = 38 [88.4 %]).
3.1. Clinical characteristics
Baseline characteristics of patients according to RDW are reported in Table 1. Patients were at low to intermediate operative risk (STS-PROM 4.0 % [IQR 2.1–6.8], EuroSCORE-II 3.8 % [IQR 2.2–6.8]). 35.8 % of patients (n = 64) had diabetes, 49.7 % (n = 86) had coronary artery disease and 72.2 % (n = 125) were in New York Heart Association (NYHA) functional class III or IV.
Table 1.
Title: Baseline characteristics.
| RDW < 15 % n = 133 |
RDW ≥ 15 % n = 43 |
p | ||
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age; years | 80.2 (77.5–84.1) | 81.2 (71.3–84.7) | 0.78 | |
| Sex; n male (%) | 90 (67.7 %) | 18 (41.9 %) | 0.004 | |
| Body mass index; kg/m2 | 26.3 (24.2–29.7) | 26.4 (22.9–33.5) | 0.89 | |
| STS-PROM; % | 3.18 (1.87–5.47) | 6.63 (4.12–10.54) | <0.001 | |
| EuroSCORE-II; % | 3.51 (2.43–5.91) | 5.52 (3.50–7.86) | 0.002 | |
| Peripheral arterial disease; n (%) | 38 (28.6 %) | 12 (27.9 %) | 1 | |
| History of stroke; n (%) | 16 (12.0 %) | 5 (11.6 %) | 1 | |
| Diabetes; n (%) | 46 (34.5 %) | 18 (41.9 %) | 0.47 | |
| On oral medication; n (%) | 28 (60.9 %) | 5 (27.8 %) | ||
| On insulin; n (%) | 12 (26.0 %) | 10 (55.6 %) | ||
| Coronary Artery Disease; n (%) | 64 (48.1 %) | 23 (53.5 %) | 0.57 | |
| 1-vessel-disease; n (%) | 24 (18.1 %) | 5 (11.6 %) | ||
| 2-vessel-disease; n (%) | 26 (19.6 %) | 10 (23.3 %) | ||
| 3-vessel-disease; n (%) | 14 (10.5 %) | 8 (18.6 %) | ||
| Atrial fibrillation; n (%) | 52 (39.1 %) | 25 (58.1 %) | 0.04 | |
| Heart failure | <0.001 | |||
| NYHA I; n (%) | 12 (9.0 %) | 1 (2.3 %) | ||
| NYHA II; n (%) | 35 (26.3 %) | 3 (7.0 %) | ||
| NYHA III; n (%) | 65 (48.9 %) | 19 (44.2 %) | ||
| NYHA IV; n (%) | 21 (15.8 %) | 20 (46.5 %) | ||
| Baseline echocardiography | ||||
| Ejection fraction; % | 55 (53–62) | 55 (50–60) | 0.40 | |
| Stroke volume index; ml/m2 | 36.0 (28.6–45.0) | 31.8 (24.6–44.1) | 0.10 | |
| Mean aortic valve gradient; mmHg | 42.0 (34.0–50.0) | 38.0 (31.0–47.0) | 0.15 | |
| Aortic valve area; cm2 | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.85 | |
| Laboratory results at baseline | ||||
| Hemoglobin; mmol/l | 13.2 g/dL (11.8–14.1) | 10.4 g/dL (9.8–12.2) | <0.001 | |
| Red blood cell count; 106/µl | 4.29 (3.92–4.67) | 3.86 (3.36–4.38) | 0.003 | |
| Thrombocyte count; 106/µl | 214 (170–263) | 213 (185–258) | 0.84 | |
| Leukocyte count; 106/µl | 7.6 (6.6–9.2) | 8.1 (6.5–10.7) | 0.51 | |
| NT-proBNP; pg/ml |
1884.5 (944.3–3575.8) |
5802.5 (2203.3–14470.3) |
<0.001 | |
| NT-proBNP; times ULN | 3.63 (1,71–8.92) | 7.86 (4.34–43.72) | <0.001 | |
| Creatinine; µmol/l | 93.5 (79.5–125.0) | 119.0 (90.0–189.0) | 0.01 | |
| GFR; ml/min | 55.5 (39.0–67.5) | 41.0 (20.7–59.6) | 0.003 | |
Categorical variables are depicted n (%) and continuous variables are depicted median (interquartile range). STS-PROM, Society of Thoracic Surgeons – Predicted Risk Of Mortality, NYHA, New York Heart Association, GFR, glomerular filtration rate.
Patients with preprocedural RDW ≥ 15 were significantly more often female (n = 43, 32.3 % vs. n = 25, 58.1 %, p = 0.004) and had significantly higher operative risk as determined by STS-PROM (3.18 % [IQR 1.87–5.91] vs. 5.52 % [IQR 3.5–7.86], p < 0.001) and EuroSCORE-II (3.51 % [IQR 2.43–5.91] vs. 5.52 [IQR 3.5–7.86], p = 0.002). In contrast, age and echocardiographic features did not differ significantly between groups (Table 1). Preprocedural hemoglobin concentration (13.2 g/dL [IQR 11.8–14.1] vs 10.4 g/dL [IQR 9.8–12.2], p < 0.001) and glomerular filtration rate (55.5 ml/min [IQR 39.0–67.5] vs. 41.0 [IQR 20.7–59.6], p = 0.003) were significantly lower in the RDW-high group. Parameters of iron and folate metabolism were only available in a minority of patients but did not differ significantly between groups (supplementary table S1). Mean corpuscular volume did not differ significantly between groups (RDW-normal: 89.7 fl [IQR 86.6–92.7] vs RDW-high: 88.9 fl [IQR 79.9–93.9], p = 0.16), but mean corpuscular hemoglobin (RDW-normal: 1.89 pg [IQR 1.82–1.94] vs RDW-high 1.75 pg [IQR 1.61–1.88], p < 0.01) and mean corpuscular hemoglobin concentration (RDW-normal: 21.1 mmol/l [IQR 20.7–21.5] vs. RDW-high 20.0 mmol/l [IQR 19.4–20.9], p < 0.001) were significantly lower in the RDW-high group.
3.2. Procedural characteristics and outcomes
53.2 % of patients received balloon-expandable valves (Edwards Sapien 3, n = 88 and Edwards S3 Ultra, n = 4), 46.2 % of patients received self-expanding valves (Medtronic Evolut R, n = 54, Medtronic Evolut Pro, n = 17, Symetis Accurate, n = 3, Accurate neo, n = 4, Accurate neo2, n = 2) and 0.6 % of patients received mechanically expanded valves (Boston Scientific Lotus, n = 1). There were no significant differences in the incidence of the VARC-3-defined periprocedural adverse events. The rates of technical success or early safety (Supplementary table S2) were high in the total population (Technical success n = 172, 97.7 %, early safety n = 130, 73.9 %) and did also not differ significantly between patients in the RDW-normal and the RDW-high group.
3.3. Mid-term outcomes according to RDW
At 1-year-follow-up, NYHA functional class and NT-proBNP were significantly higher in patients in the RDW-high group (NT-proBNP 884.5 pg/ml [IQR 367.8–2099.5] vs. 1167.0 [IQR 320.5–3102.5] or 1.79 times ULN [IQR 0.79–3.76] vs. 2.36 times ULN [IQR 0.43–4.52], respectively, p = 0.004, Fig. 1). Relative decrease of NT-proBNP did not differ significantly between groups (at 1 month: RDW-normal: 35.0 % [IQR 25.0 %-67.0 %] of the serum NT-proBNP concentration before TAVI, RDW-high: 42.5 % [IQR 13.75 %-58.75 %] in the RDW-high group, p = 0.79), and there was no significant change between 1 month and 12 months (RDW-normal: 48.1 % [IQR 11.2 %-94.1 %], RDW-high: 46.2 % [IQR 23.7 %-78.3 %], p = 0.163 for comparison between 1 and 12 months after TAVI, respectively, supplementary figure S3). Hemodynamic performance of the valve prosthesis and left ventricular ejection fraction did not differ significantly between groups (Supplementary figure S4 A-D), however, stroke volume index and markers of diastolic dysfunction differed significantly (Supplementary figure S4 E-G).
Fig. 1.
Title: Heart failure status after 12 months. Caption: A, NYHA functional class at 12 months follow-up. B, NT-proBNP in pg/ml at 12 months follow-up.
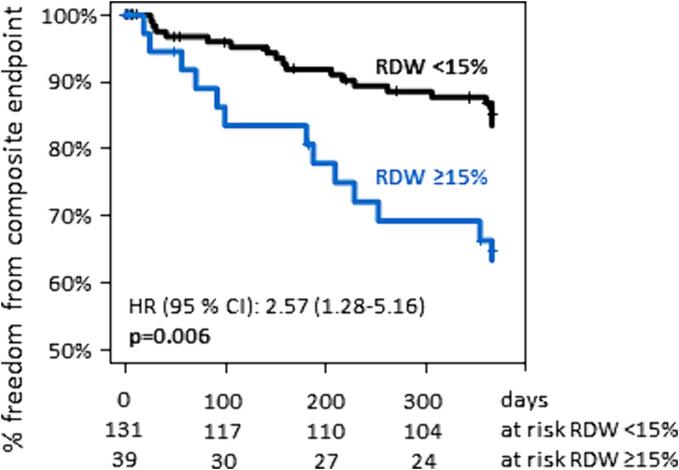
All-cause mortality was low in the overall group and did not differ significantly between groups (RDW-normal 7.9 % [n = 9] vs. RDW-high: 9.4 % [n = 3], p = 0.79). Cardiovascular and valve-related mortality were also not significantly different (0.8 % [n = 1] vs. 2.3 % [n = 1], p = 0.40, respectively). However, incidence of the composite endpoint of cardiovascular/ valve-related mortality and cardiovascular/ heart failure/ valve-related hospitalization was significantly higher in patients with elevated RDW at baseline (RDW-normal: 15.3 % [n = 20] vs. RDW-high: 33.3 % [n = 13], p = 0.006, Fig. 2).
Fig. 2.
Title: Incidence of composite endpoint. Caption: Incidence of composite endpoint comprising cardiovascular/ valve-related death and cardiovascular/ heart failure-related/ valve-related hospitalization. HR, hazard ratio, CI, confidence interval.
3.4. Adjustment for differences in baseline characteristics
Multivariable Cox regression of all variables differing significantly between preprocedural RDW groups was performed. In a multivariate model accounting for differences in age, sex, STS-PROM, EuroSCORE-II, incidence of atrial fibrillation, preprocedural hemoglobin, NT-proBNP, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration and GFR, RDW remained an independent predictor of the composite endpoint (hazard ratio per percentage point RDW 1.33 [95 % confidence interval 1.15–1.54], p < 0.001, hazard ratio for RDW-high vs. RDW-normal: 2.57 [95 % confidence interval 1.28–5.16], p < 0.001, Table 2).
Table 2.
Title: Multivariable analysis of predictors of composite endpoint.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95 % CI) | p | HR (95 % CI) | p | |
| Age | 0.99 (0.94–1.04) | 0.68 | ||
| Male sex | 1.35 (0.65–2.85) | 0.42 | ||
| STS-PROM | 1.07 (1.01–1.13) | 0.02 | 0.23 | |
| EuroSCORE-II | 1.068 (1.00-0.1.14) | 0.04 | 0.82 | |
| Atrial fibrillation | 0.67 | |||
| Hemoglobin baseline | 0.14 | |||
| Red blood cell count | 0.94 | |||
| NT-proBNP baseline | 0.11 | |||
| RDW baseline | 1.33 (1.15–1.53) | <0.001 | 1.33 (1.15–1.53) | <0.001 |
| MCH baseline | 0.15 | |||
| MCHC baseline | 0.60 (0.42–0.87) | 0.006 | 0.18 | |
| GFR baseline | 0.92 | |||
| Stroke volume index baseline | 0.982 (0.962–1.002) | 0.08 | 0.23 | |
| E/E’ baseline | 0.55 | |||
| TRvmax baseline | 0.94 | |||
HR, hazard ratio, CI, confidence interval, STS-PROM, Society of Thoracic Surgeons Predicted Risk Of Mortality, MCH, mean corpuscular hemoglobin, MCHC, mean corpuscular hemoglobin concentration, GFR, glomerular filtration rate, RDW, Red cell Distribution Width, TRvmax, maximum velocity of tricuspid regurgitation by doppler echocardiography.
4. Discussion
To identify patients at risk for insufficient functional improvement after TAVI remains an important issue. Although the association of elevated RDW with all-cause death has been demonstrated for a variety of cardiovascular conditions, e.g. heart failure [9], [10], coronary artery disease [11], [12] and patients at very high operative risk after TAVI [13], its connection to functional status is less clear. Our study identifies RDW as a predictor of insufficient functional improvement after transfemoral TAVI in a contemporary intermediate-risk cohort.
Technical success was high and periprocedural adverse events were rare with no significant difference between RDW-normal and RDW-high groups. Although there was no significantly higher mortality in patients with elevated RDW, these patients had a higher incidence of the composite endpoint of cardiovascular/ valve-related death and cardiovascular, heart failure or valve-related hospitalization. Higher STS-PROM and EuroSCORE-II as well as higher serum creatinine and lower hemoglobin levels at baseline in patients with high RDW signified that these patients suffered from more severe comorbidities. Nonetheless, RDW remained the only independent predictor of our composite endpoint in multivariable analysis, suggesting additional and independent predictive value.
4.1. Pathophysiological considerations
There has been some research into the pathophysiological relationships between RDW and cardiovascular outcomes: As erythrocyte volumes decrease across their lifespan, a higher RDW typically reflects impaired erythrocyte homeostasis with either impaired erythropoiesis or abnormally prolonged red blood cell survival [8]. This can be seen with e.g. poor nutritional status, elevated levels of oxidative stress or a proinflammatory state [16]. Anisocytosis, i.e. elevated RDW, and consequent alterations to the erythrocyte membrane [18] have also been implicated in actively promoting atherosclerosis [16] via intraplaque hemorrhage and impaired blood flow in the microcirculation [18], [19], [20].
The importance of the presence of Clonal Hematopoiesis of Indeterminate Potential (CHIP), i.e. somatic mutations in genes recurrently mutated in myeloid malignancies without a present overt hematologic malignancy, has been thoroughly evaluated in hematological disorders [21] Also, patients with CHIP have been shown to have an increased risk of cardiovascular mortality independent of traditional risk factors, [22], [23] both generally and also specifically after TAVI [24]. Large scale evaluation of CHIP significance is cumbersome due to costs and complexity of sequencing. Notably, in large cohorts, all-cause mortality was only significantly increased in patients with CHIP also having elevated RDW, and carriers of CHIP were shown to have significantly higher RDW [21].
4.2. Previous results
In previous works, very high- or prohibitive risk cohorts after TAVI have been examined and both absolute preprocedural values [13], [14], [25], [26] as well as rise after TAVI [27] have been shown to predict all-cause mortality. However, as RDW is elevated both in elderly people and in a variety of serious conditions [10], [15], and mainly all-cause mortality was examined, clear attribution of the effect to cardiovascular events could not be demonstrated. Moreover, procedural risk as determined by STS-PROM was higher than in our contemporary cohort, probably because earlier device iterations possibly more prone to bleeding complications [28] were used, and data on severity of heart failure symptoms was not reported. In our cohort, albeit NT-proBNP was reduced by TAVI in a comparable fashion in both groups, it was shown to be significantly higher at follow-up in patients with elevated RDW and NYHA class was worse. Persistently elevated NT-proBNP despite comparable prosthesis performance might reflect more severe cardiac damage in RDW—high patients.
Moreover, our composite endpoint focused on cardiovascular events and comprised hospitalization, which is especially important from a patient perspective [29].
4.3. Clinical relevance
As RDW is automatically determined in every standard blood count, it can be taken into account without additional effort. Provided that our findings hold true after prospective validation, RDW could be of use when counseling patients, planning the TAVI procedure and scheduling follow-up visits: As functional recovery is a very important procedural goal for patients [29], considering RDW might help when counseling elderly patients on the decision to pursue either an interventional approach or seeking best supportive care in advanced aortic stenosis. When planning the procedure, patients with elevated RDW should be monitored more closely, and seem to be less suitable for a ‘fast-track’ TAVI approach [30]. Most importantly, follow-up after TAVI should be more closely in patients with elevated RDW to recognize signs of progressive heart failure as early as possible and to avoid hospitalizations for decompensated heart failure.
4.4. Limitations
This paper has several limitations. First, sample size is relatively small and single-centered, limiting our ability to generate a predictive model. Second, CHIP status was not available in our cohort. Third, a more detailed objective quantitation of heart failure symptoms, e.g. by 6-minute walking tests of Kansas City Cardiac Questionnaire would have been helpful. Fourth, as RDW values differ among laboratory instruments [16], analysis in several centers is warranted.
5. Conclusion
To conclude, elevated preprocedural RDW is a useful parameter to identify patients at risk for unsatisfactory functional outcome after TAVI and seems to be an easily accessible and low-cost parameter to guide intensity of outpatient follow-up. These results mandate evaluation in larger cohorts and further research into the connection between hematopoiesis and cardiovascular outcomes.
CRediT authorship contribution statement
Georg Stachel: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Madlen Jentzsch: Writing – review & editing, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Michelle Oehring: Writing – review & editing, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation. Marios Antoniadis: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation. Sebastian Schwind: Methodology, Conceptualization. Thilo Noack: Writing – review & editing, Validation, Resources, Project administration, Investigation, Data curation. Uwe Platzbecker: Writing – review & editing, Supervision, Methodology, Data curation, Conceptualization. Michael Borger: Writing – review & editing, Supervision, Resources, Project administration, Methodology. Ulrich Laufs: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Conceptualization. Karsten Lenk: Writing – review & editing, Supervision, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
Thilo Noack, MD: speaker’s honoraria and/ or consulting fees from Edwards Lifesciences and Abbott Vascular Michael Borger, MD, PhD: speaker’s honoraria paid to his hospital on his behalf from Edwards Lifesciences, Medtronic, Artivion and Abbott Vascular.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101383.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 2.Patel K.P., Treibel T.A., Scully P.R., et al. Futility in Transcatheter Aortic Valve Implantation: A Search for Clarity. Interv Cardiol. 2022;17:e01. doi: 10.15420/icr.2021.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genereux P., Pibarot P., Redfors B., et al. Evolution and Prognostic Impact of Cardiac Damage After Aortic Valve Replacement. Journal of the American College of Cardiology. 2022;80:783–800. doi: 10.1016/j.jacc.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Freitas-Ferraz A.B., Nombela-Franco L., Urena M., et al. Transcatheter aortic valve replacement in patients with paradoxical low-flow, low-gradient aortic stenosis: Incidence and predictors of treatment futility. International Journal of Cardiology. 2020;316:57–63. doi: 10.1016/j.ijcard.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Lindman B.R., Alexander K.P., O'Gara P.T., Afilalo J. Futility, benefit, and transcatheter aortic valve replacement. JACC Cardiovascular Interventions. 2014;7:707–716. doi: 10.1016/j.jcin.2014.01.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raess P.W., van de Geijn G.J., Njo T.L., et al. Automated screening for myelodysplastic syndromes through analysis of complete blood count and cell population data parameters. Am J Hematol. 2014;89:369–374. doi: 10.1002/ajh.23643. [DOI] [PubMed] [Google Scholar]
- 7.Pilling L.C., Atkins J.L., Kuchel G.A., Ferrucci L., Melzer D. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PloS One. 2018;13:e0203504. doi: 10.1371/journal.pone.0203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vucinic V., Ruhnke L., Sockel K., et al. The diagnostic red blood cell distribution width as a prognostic factor in acute myeloid leukemia. Blood Adv. 2021;5:5584–5587. doi: 10.1182/bloodadvances.2021005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felker G.M., Allen L.A., Pocock S.J., et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. Journal of the American College of Cardiology. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 10.Forhecz Z., Gombos T., Borgulya G., Pozsonyi Z., Prohaszka Z., Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. American Heart Journal. 2009;158:659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Dabbah S., Hammerman H., Markiewicz W., Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. The American Journal of Cardiology. 2010;105:312–317. doi: 10.1016/j.amjcard.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Moriya S., Wada H., Iwata H., et al. Red Cell Distribution Width Predicts Long-Term Cardiovascular Outcomes in Patients with Chronic Coronary Syndrome. International Heart Journal. 2022;63:1041–1047. doi: 10.1536/ihj.22-304. [DOI] [PubMed] [Google Scholar]
- 13.Valenti A.C., Vitolo M., Manicardi M., et al. Red blood cell distribution width in patients undergoing transcatheter aortic valve implantation: Implications for outcomes. Int J Clin Pract. 2021;75:e14153. doi: 10.1111/ijcp.14153. [DOI] [PubMed] [Google Scholar]
- 14.Szekely Y., Finkelstein A., Bazan S., et al. Red blood cell distribution width as a prognostic factor in patients undergoing transcatheter aortic valve implantation. Journal of Cardiology. 2019;74:212–216. doi: 10.1016/j.jjcc.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Allen L.A., Felker G.M., Mehra M.R., et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16:230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvagno G.L., Sanchis-Gomar F., Picanza A., Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 17.Genereux P., Piazza N., Alu M.C., et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. Journal of the American College of Cardiology. 2021;77:2717–2746. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Tziakas D.N., Chalikias G.K., Stakos D., et al. Independent and additive predictive value of total cholesterol content of erythrocyte membranes with regard to coronary artery disease clinical presentation. International Journal of Cardiology. 2011;150:22–27. doi: 10.1016/j.ijcard.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Kolodgie F.D., Gold H.K., Burke A.P., et al. Intraplaque hemorrhage and progression of coronary atheroma. The New England Journal of Medicine. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 20.Patel K.V., Mohanty J.G., Kanapuru B., Hesdorffer C., Ershler W.B., Rifkind J.M. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol. 2013;765:211–216. doi: 10.1007/978-1-4614-4989-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaiswal S., Fontanillas P., Flannick J., et al. Age-related clonal hematopoiesis associated with adverse outcomes. The New England Journal of Medicine. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein A., Metzeler K., Kubasch A.S., et al. Clonal hematopoiesis and cardiovascular disease: deciphering interconnections. Basic Research in Cardiology. 2022;117:55. doi: 10.1007/s00395-022-00969-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohme M., Desch S., Rosolowski M., et al. Impact of Clonal Hematopoiesis in Patients With Cardiogenic Shock Complicating Acute Myocardial Infarction. Journal of the American College of Cardiology. 2022;80:1545–1556. doi: 10.1016/j.jacc.2022.08.740. [DOI] [PubMed] [Google Scholar]
- 24.Mas-Peiro S., Hoffmann J., Fichtlscherer S., et al. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. European Heart Journal. 2020;41:933–939. doi: 10.1093/eurheartj/ehz591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collas V.M., Paelinck B.P., Rodrigus I.E., Vrints C.J., Van Craenenbroeck E.M., Bosmans J.M. Red cell distribution width improves the prediction of prognosis after transcatheter aortic valve implantation. European Journal of Cardio-Thoracic Surgery : Official Journal of the European Association for Cardio-Thoracic Surgery. 2016;49:471–477. doi: 10.1093/ejcts/ezv152. [DOI] [PubMed] [Google Scholar]
- 26.Hellhammer K., Zeus T., Verde P.E., et al. Red cell distribution width in anemic patients undergoing transcatheter aortic valve implantation. World J Cardiol. 2016;8:220–230. doi: 10.4330/wjc.v8.i2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aung N., Dworakowski R., Byrne J., et al. Progressive rise in red cell distribution width is associated with poor outcome after transcatheter aortic valve implantation. Heart. 2013;99:1261–1266. doi: 10.1136/heartjnl-2013-303910. [DOI] [PubMed] [Google Scholar]
- 28.Stortecky S., Franzone A., Heg D., et al. Temporal trends in adoption and outcomes of transcatheter aortic valve implantation: a SwissTAVI Registry analysis. Eur Heart J Qual Care Clin Outcomes. 2019;5:242–251. doi: 10.1093/ehjqcco/qcy048. [DOI] [PubMed] [Google Scholar]
- 29.Beishuizen S.J.E., Festen S., van der Werf H.W., de Graeff P., van Munster B.C. Was it worth it? Benefits of transcatheter aortic valve implantation from a patient's perspective. Journal of the American Geriatrics Society. 2021;69:2605–2611. doi: 10.1111/jgs.17216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopra M., Luk N.H.V., De Backer O., Sondergaard L. Simplification and optimization of transcatheter aortic valve implantation - fast-track course without compromising safety and efficacy. BMC Cardiovascular Disorders. 2018;18:231. doi: 10.1186/s12872-018-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.