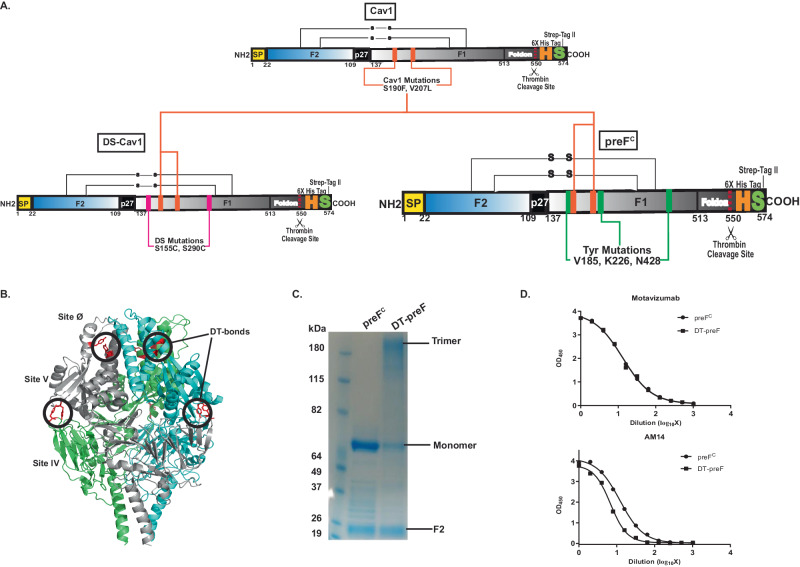
Fig. 2. Design and characterization of dityrosine crosslinked DT-preF molecule.
A TOP: RSV F parent molecule (Cav1) with Cav1 mutations indicated; Bottom Left: DS-Cav1 successor of Cav1 parent with additional DS mutations indicated. Bottom Right: preFC with tyrosine mutations indicated for dityrosine pairing. Note: K226Y pairs with endogenous tyrosine Y198. Amino acid numbering is indicated below the stick diagram. The “H”, “S”, and scissors represent 6X His tag, Strep-II tag, and Thrombin cleavage site, respectively. B Crystal structure of DS-Cav1 protein with preFC mutations modeled on the structure. DT bond locations are circled indicating the intramolecular crosslink preserving site Ø (5C4, D25) and the intermolecular crosslink preserving the site IV/V interface (AM14). C Coomassie Blue Protein staining of SDS-PAGE separated preFC and DTpre-F under reducing conditions before and after crosslinking. Monomer, trimer, and F2 are indicated. Similar gel shifts have been observed consistently (>100 independent experiments). D ELISA binding curves of preFC and DT-preF using primary antibodies Motavizumab and AM14 before and after crosslinking. Source data are provided as a Source Data file, the average data from 2 different crosslinking experiments is graphed.