Figure 2 .
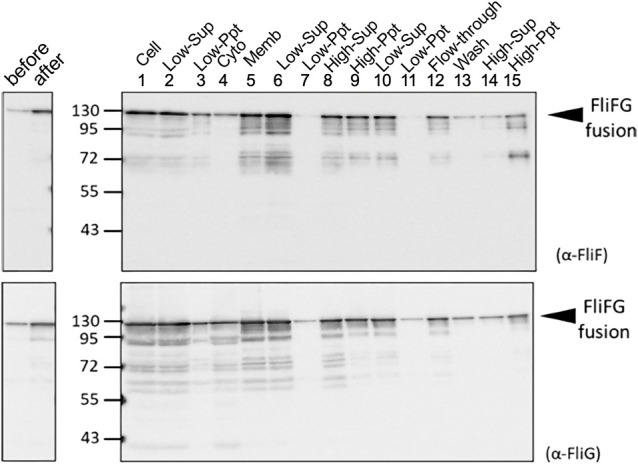
Purification of FliFG fusion protein. E. coli cells expressing FliFG fusion protein were harvested and suspended in the buffer. The cells (1: Cell) were sonicated, and the pellet (3: Low-Ppt) and supernatant (2: Low-Sup) were obtained using low-speed centrifugation. The supernatant was centrifuged at high speed to obtain the supernatant (4: Cyto) and the pellet (5: Memb). The membrane fraction was solubilized using a detergent, and the supernatant (6) and pellet (7) were obtained using low-speed centrifugation. The obtained supernatant (6) was centrifuged at high speed to obtain the supernatant (8) and the pellet (9). This pellet of (9) was suspended and centrifuged again at low speed to further separate the supernatant (10) and the pellet (11). The supernatant (10) was loaded in the column for his-tag. The flow-through (12) was recovered, washed with buffer (13), and eluted with imidazole. The eluted fraction was centrifuged at high speed to separate the supernatant (14) and the pellet (15). The proteins were separated using SDS-PAGE and detected using immunoblotting using anti-FliF (A) and anti-FliG (B) antibodies.
