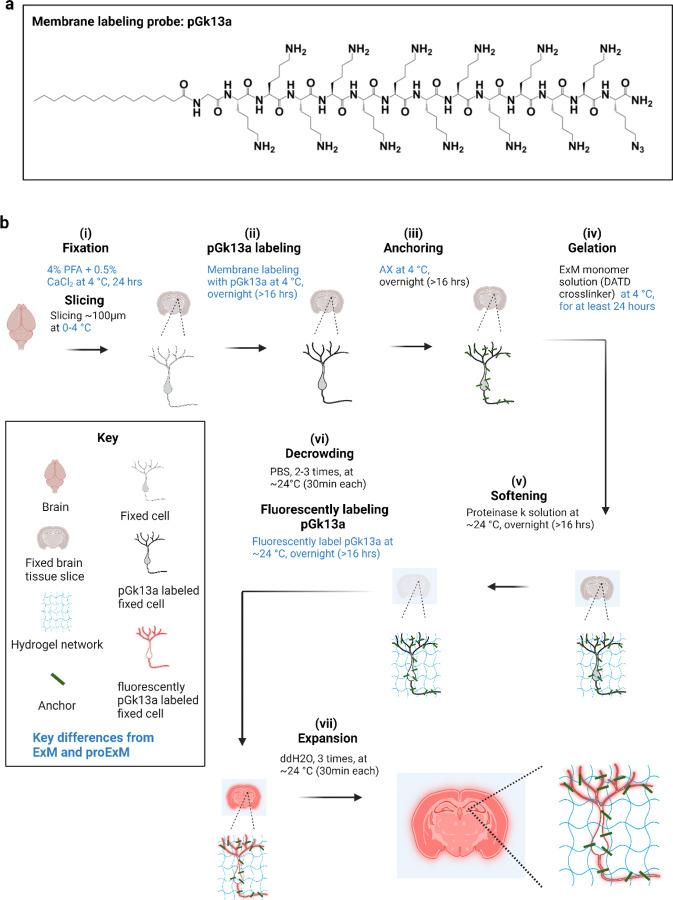
Figure 1. Ultrastructural membrane expansion microscopy (umExM) concept and workflow.
umExM is a modified form of expansion microscopy with a custom-designed amphiphilic membrane labeling probe (termed pGk13a). (a) Chemical structure of pGk13a. The probe does not contain any fluorophore but has an azide to bind a fluorophore later. (b) umExM workflow. Blue-colored fine text highlight key differences from ExM1 and proExM4, whereas black fine text highlight the same steps as ExM and proExM. (b.i) A specimen is perfused and chemically fixed with 4% paraformaldehyde (PFA) + 0.5% calcium chloride (CaCl2) at 4 °C for 24 hours. The brain is sliced on a vibratome to 100 μm thickness at 0–4 °C. (b.ii) The specimen is treated with pGk13a (structure is depicted in (a)) at 4 °C overnight (unless otherwise noted, overnight means >16 hours). (b.iii) The specimen is treated with acrylic acid N-hydroxysuccinimide ester (AX) at 4 °C overnight. (b.iv) The specimen is embedded in an expandable hydrogel (made with N,N’-Diallyl-L-tartardiamide (DATD) crosslinker4) at 4 °C for at least 24 hours. (b.v) The sample (specimen-embedded hydrogel) is chemically softened with enzymatic cleavage of proteins (i.e., non-specific cleavage with proteinase K) at room temperature (~24 °C) overnight. The probe is not digested during proteinase K treatment since it is composed of D-amino acids. (b.vi) Then, the sample is treated with 1x phosphate-buffered saline (PBS) to partially expand it. The pGk13a, that is anchored to the gel matrix, is fluorescently labeled via click-chemistry (i.e., DBCO-fluorophore) at room temperature, overnight. (b.vii) The sample is expanded with water at room temperature for 1.5 hours (exchanging water every 30 minutes).