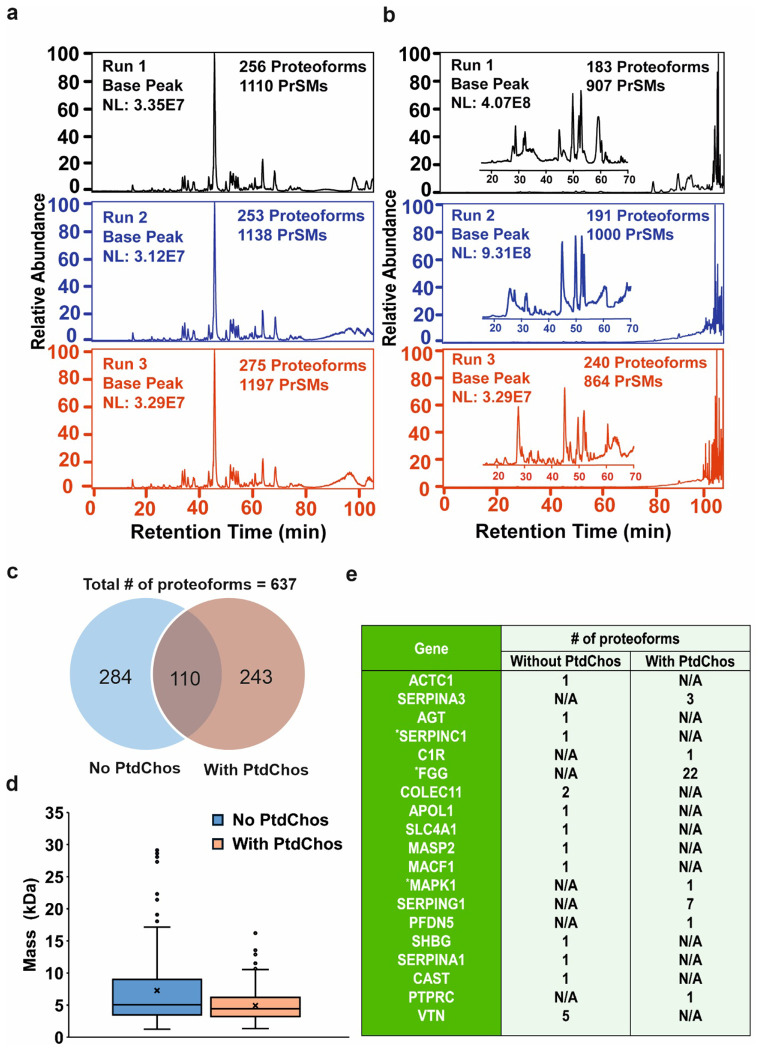
Fig. 3. Base peak chromatograms and proteoform analysis of protein corona samples.
a, Base peak chromatogram of eluted protein corona without PtdChos and b, with PtdChos in healthy human plasma after RPLC-MS/MS analyses. Two protein corona samples were prepared in parallel and analyzed by RPLC-MS/MS, with each sample measured in triplicate. c, The number of proteoform identifications in each sample and the overlap of proteoform identifications between the two samples. d, Mass distribution of proteoforms between the two samples, with the cross sign representing the mean proteoform mass in each sample (Center line—median; box limits contain 50% of data; upper and lower quartiles, 75 and 25%; maximum—greatest value excluding outliers; minimum—least value excluding outliers; outliers—more than 1.5 times of the upper and lower quartiles). e, Summary of some disease-related protein biomarkers identified by top-down proteomics. The Genes were determined according to the information in the Human Protein Atlas (https://www.proteinatlas.org/) and three genes labelled by * represent FDA approved drug target.