Summary
In light of escalating sustainability concerns, addressing catalyst usage and waste production challenges becomes crucial. Here, we introduce a robust protocol for crafting recyclable polystyrene-supported primary amines, providing a promising solution via heterogeneous catalysis. The protocol details immobilization onto insoluble resins through ester, ether, or amide bonds, facilitating the synthesis of heterogeneous catalysts with diverse organic components.
For complete details on the use and execution of this protocol, please refer to Kanger et al.1
Subject areas: Chemistry, Material sciences
Graphical abstract

Highlights
-
•
Synthesis of insoluble heterogeneous aminocatalysts
-
•
Different general approaches to immobilization onto various resins
-
•
Compatibility with numerous amino acids
-
•
Infrared spectroscopy, scanning electron microscopy, and elemental analysis
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
In light of escalating sustainability concerns, addressing catalyst usage and waste production challenges becomes crucial. Here, we introduce a robust protocol for crafting recyclable polystyrene-supported primary amines, providing a promising solution via heterogeneous catalysis. The protocol details immobilization onto insoluble resins through ester, ether, or amide bonds, facilitating the synthesis of heterogeneous catalysts with diverse organic components.
Before you begin
In the relentless pursuit of efficient natural resource utilization, the global focus on sustainability is underscored by the United Nations' Sustainable Development Goals.2 Of particular significance are concerns related to sustainable industrialization, judicious consumption, waste reduction, and the reusability of materials. Within the scientific community, efforts to enhance the environmental sustainability of chemical processes have gained momentum. Organocatalysis,3,4 encompassing primary amine catalysis,5 has emerged as a powerful tool in asymmetric synthesis, yet industry faces a significant hurdle in catalyst regeneration.6 To address this limitation and promote widespread application, the potential of heterogeneous catalysis has been explored.1,7 The proposed method involves immobilizing catalysts on insoluble resin, presenting a process characterized by operational simplicity. After the reaction’s completion, a straightforward filtration step allows for the easy recovery of the desired product from the supernatant. The utilized catalyst can then undergo a simple washing process with various solvents, restoring its catalytic efficiency. The regenerated catalyst, following a drying step, can be reintroduced in subsequent reactions involving fresh batches of initial starting material. The fine-tuning of immobilization techniques not only ensures recyclability but also opens avenues for diverse chiral organocatalysts on solid support.
This comprehensive protocol provides step-by-step guidance for the synthesis and immobilization of recyclable organocatalysts on polystyrene resins. The incorporation of various amino acid derivatives onto the resin is monitored using IR spectrometry, and catalyst loadings are estimated through elemental analysis, ensuring reproducibility and reliability in catalyst preparation. The detailed procedures cover diverse immobilization strategies, offering flexibility for researchers to choose the most suitable approach for their specific catalytic requirements.
This protocol details the synthesis and immobilization of heterogeneous organocatalysts on divinylbenzene cross-linked polystyrene resins for sustainable and recyclable catalysis. At the project’s initiation, various strategies for immobilizing primary amines onto the resin were considered. In the first strategy, the Merrifield resin was employed and functionalized with protected α-amino acids, resulting in catalysts with a loading range of 0.59–0.64 mmol/g. For the second approach, the Wang resin was utilized, and the unreacted active sites were end-capped with acetic anhydride to form ester-linked supported catalysts. Additionally, a nucleophilic substitution reaction with sodium hydride and potassium iodide was employed for direct attachment of Boc-protected quinine and tyrosine derivatives to the Merrifield resin, yielding catalysts with loadings of 0.6 mmol/g and 0.34 mmol/g, respectively. The third method involved coupling protected α-amino acids with (aminomethylated)polystyrene using peptide synthesis conditions, and subsequent blocking of free primary amines through amide formation or alkylation with acetic anhydride, pivaloyl chloride, and methyl iodide, resulting in catalysts with loadings in the range of 0.38 mmol/g–0.59 mmol/g.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Deuterated chloroform | Deutero | Cas: 865-49-6 |
| Dichloroethane | Penta | Cas: 107-06-2 |
| Dichloromethane | Honeywell | Cas: 75-09-2 |
| Toluene | Keemiakaubandus AS | Cas: 108-88-3 |
| Acetonitrile | Honeywell | Cas: 75-05-8 |
| Cyclopentyl methyl ether | Thermo Fisher Scientific | Cas: 5614-37-9 |
| Methanol | Keemiakaubandus AS | Cas: 67-56-1 |
| N,N-dimethylformamide | Thermo Fisher Scientific | Cas: 68-12-2 |
| Isopropyl alcohol | Thermo Fisher Scientific | Cas: 67-63-0 |
| Tetrahydrofuran | Thermo Fisher Scientific | Cas: 109-99-9 |
| Diethyl ether | Honeywell | Cas: 60-29-7 |
| Petroleum ether (bp: 40°C–60°C) | Honeywell | Cas: 8032-32-4 |
| Ethyl acetate | Keemiakaubandus AS | Cas: 141-78-6 |
| Hexane | Honeywell | Cas: 110-54-3 |
| Trifluoroacetic acid | Apollo Scientific | Cas: 76-05-1 |
| Triethylamine | Fisher Chemical | Cas: 121-44-8 |
| Piperidine | Alfa Aesar | Cas: 110-89-4 |
| Pyridine | Acros Organics | Cas: 110-86-1 |
| 4-dimethylaminopyridine | Acros Organics | Cas: 1122-58-3 |
| Acetic anhydride | Reachim | Cas: 108-24-7 |
| Para-nitrobenzoic acid | Honeywell | Cas: 62-23-7 |
| Potassium fluoride | Sigma-Aldrich | Cas: 7789-23-3 |
| Potassium carbonate | Lachner | Cas: 584-08-7 |
| 1-Hydroxybenzotriazole hydrate | Sigma-Aldrich | Cas: 123333-53-9 |
| N,N′-diisopropylcarbodiimide | Sigma-Aldrich | Cas: 693-13-0 |
| Triphenylphosphine | Fluorochem | Cas: 603-35-0 |
| Diisopropyl azodicarboxylate | Thermo Fisher Scientific | Cas: 2446-83-5 |
| Diphenylphosphoryl azide | Sigma-Aldrich | Cas: 26386-88-9 |
| Boron tribromide (1 M solution in DCM) | Sigma-Aldrich | Cas: 10294-33-4 |
| Di-tert-butyl dicarbonate | Acros Organics | Cas: 24424-99-5 |
| Iodine | Reachim | Cas: 7553-56-2 |
| Sodium hydride (60% in mineral oil) | Sigma-Aldrich | Cas: 7646-69-7 |
| Sodium thiosulfate | Merck | Cas: 7772-98-7 |
| Thionyl chloride | Acros Organics | Cas: 7719-09-7 |
| Butyl amine | Sigma-Aldrich | Cas: 109-73-9 |
| Pivaloyl chloride | Sigma-Aldrich | Cas: 3282-30-2 |
| Methyl iodide | Sigma-Aldrich | Cas: 74-88-4 |
| Boc-(L)-α-tert-butylglycine | Sigma-Aldrich | Cas: 62965-35-9 |
| Boc-(L)-alanine | Orpegen | Cas: 15761-38-3 |
| Boc-(L)-leucine | Alfa Aesar | Cas: 13139-15-6 |
| Boc-O-benzyl-(L)-serine | Orpegen | Cas: 23680-31-1 |
| Boc-(L)-methionine | Orpegen | Cas: 2488-15-5 |
| Boc-(L)-2-propargylglycine | Sigma-Aldrich | Cas: 63039-48-5 |
| Fmoc-(L)-phenylalanine | Sigma-Aldrich | Cas: 35661-40-6 |
| Fmoc-(L)-isoleucine | Fluorochem | Cas: 71989-23-6 |
| Fmoc-(L)-valine | Sigma-Aldrich | Cas: 68858-20-8 |
| Fmoc-(L)-alanine | Orpegen | Cas: 35661-39-3 |
| (L)-phenylalanine | Strem | Cas: 63-91-2 |
| (L)-α-phenylglycine | Lancaster | Cas: 2935-35-5 |
| Quinine | Sigma-Aldrich | Cas: 130-95-0 |
| Merrifield resin LL | Sigma-Aldrich |
f = 1.02 mmol/g (functionalization level provided by supplier), 100–200 mesh, 1% DVB |
| (Aminomethylated)polystyrene | Sigma-Aldrich |
f = 1.2 mmol/g (functionalization level provided by supplier), 70–90 mesh, 1% DVB |
| Wang resin | Iris Biotech |
f = 1.2 mmol/g (functionalization level provided by supplier), 100–200 mesh, 1% DVB |
| Silica gel | Kieselgel | 40−63 μm |
| Software and algorithms | ||
| ChemDraw Professional 20.0 | PerkinElmer | https://www.perkinelmer.com/category/chemdraw |
| Other | ||
| Thin layer chromatography (TLC) plate | Merck | DC-Alufolien, Kieselgel F254 |
| Shaker | Heidolph | UNIMAX 1010 |
| Thermoregulated shaker | Heidolph | UNIMAX 1010 with heating module Inkubator 1000) |
| Analytical balance | KERN Precisa Mettler Toledo |
ABT 220-4NM 220 M SCS PB1502-S/FACT |
| Magnetic stirrer (0–1500 rpm) | IKA | RET basic |
| Automated flash chromatography instrument | Biotage | Isolera Prime ISO-PSV |
| Rotary evaporator | Heidolph Büchi |
Laborota 4000 R-300; B-300 Base; Interface I-100; Vacuum Pump V-100 |
| High vacuum pump | Edwards | RV5 |
| Nuclear magnetic resonance spectrometer | Bruker | Avance III 400 MHz |
| IR spectrometer | Bruker | Tensor 27 FT-IR |
| Elemental analyzer | Elementar | Vario-Micro V2.2.0 CHNS |
| Scanning electron microscope (SEM) | Zeiss | FEG-SEM Ultra-55 |
| Liquid chromatography-mass spectrometer (LC-MS) | Agilent | G4220A (1290 Bin Pump); G1330B (1290 Thermostat); G4226B (1290 Sampler); G1316C (1290 TCC); 6540 UHD Accurate-Mass Q-TOF (ESI ionization) |
Materials and equipment
Synthesis of the heterogeneous catalysts 3a – 3c
| Reagent | Concentration | Amount |
|---|---|---|
| Merrifield resin | 0.204 mmol | 200 mg |
| Boc-(L)-alanine 1a | 0.306 mmol | 58 mg |
| Boc-(L)-leucine 1b | 0.306 mmol | 71 mg |
| Boc-O-benzyl-(L)-serine 1c | 0.306 mmol | 90 mg |
| Potassium fluoride | 0.612 mmol | 36 mg |
| N,N-dimethylformamide | 0.136 M | 1.5 mL |
CRITICAL: Potassium fluoride is toxic if swallowed, inhaled, or absorbed through the skin. It can cause severe irritation and burns to the eyes, skin, and respiratory tract. It reacts violently with strong acids, liberating highly toxic hydrogen fluoride gas.
Synthesis of the heterogeneous catalysts 6a – 6d
| Reagent | Concentration | Amount |
|---|---|---|
| Wang resin | 0.24 mmol | 200 mg |
| Fmoc-(L)-phenylalanine 4a | 0.48 mmol | 186 mg |
| Fmoc-(L)-isoleucine 4b | 0.48 mmol | 170 mg |
| Fmoc-(L)-valine 4c | 0.48 mmol | 163 mg |
| Fmoc-(L)-alanine 4d | 0.48 mmol | 149 mg |
| Hydroxybenzotriazole | 0.48 mmol | 65 mg |
| N,N-diisopropylcarbodiimide | 0.24 mmol | 38 μL |
| 4-Dimethylaminopyridine | 0.024 mmol | 3 mg |
| Acetic anhydride | 0.48 mmol | 45 μL |
| Pyridine | 0.48 mmol | 39 μL |
| Dichloromethane | 0.09 M | 2.7 mL |
| N,N-dimethylformamide | 0.8 M | 0.3 mL |
CRITICAL: Hydroxybenzotriazole may cause skin and eye irritation, thus direct contact with the skin or eyes should be avoided. Heating or burning hydroxybenzotriazole may produce toxic fumes, and inhalation of these fumes should be avoided. Hydroxybenzotriazole is harmful to aquatic life, thus releasing it into water systems should be avoided. N,N-diisopropylcarbodiimide (DIC) is highly flammable, keep it away from open flames, sparks, and heat sources. It can cause irritation to the eyes, skin, and respiratory system, thus direct contact with the eyes, skin, or inhalation of vapors should be avoided. DIC is reactive and should be handled carefully to prevent unwanted reactions. It reacts violently with water, releasing toxic fumes of carbon dioxide. 4-Dimethylaminopyridine (DMAP) may cause irritation to the eyes, skin, and respiratory system, thus direct contact with the eyes, skin, or inhalation of vapors should be avoided. DMAP is toxic if ingested, thus ingestion should be avoided. Acetic anhydride is corrosive and can cause severe burns and irritation to the skin, eyes, and respiratory tract. Inhalation of vapors or ingestion can be harmful and may cause respiratory and gastrointestinal issues. It may cause irritation upon contact with the skin, eyes, and mucous membranes. Acetic anhydride is highly flammable and poses a fire hazard. It may form explosive mixtures in the air. It reacts violently with water, liberating acetic acid, thus care should be taken to avoid moisture. Pyridine is toxic and can cause harmful effects if swallowed, inhaled, or absorbed through the skin. It may cause irritation to the eyes, skin, and respiratory tract upon contact. Pyridine is highly flammable and poses a fire hazard. It reacts with strong oxidizing agents and can form explosive mixtures.
Synthesis of the heterogeneous catalyst 12
| Reagent | Concentration | Amount |
|---|---|---|
| Merrifield resin | 0.204 mmol | 200 mg |
| tert-butyl ((S)-(6-hydroxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)carbamate 10 | 0.24 mmol | 100 mg |
| Potassium iodide | 0.02 mmol | 3.4 mg |
| Sodium hydride (60% in mineral oil) | 0.31 mmol | 12 mg |
| N,N-dimethylformamide | 0.051 M | 4 mL |
CRITICAL: Sodium hydride reacts violently with water, releasing flammable hydrogen gas, thus it is crucial to keep it away from moisture. Sodium hydride can ignite spontaneously in the presence of moisture or air, leading to a fire hazard. It can cause corrosion and burns upon direct contact with the skin, eyes, and mucous membranes.
Synthesis of the heterogeneous catalyst 18
| Reagent | Concentration | Amount |
|---|---|---|
| Merrifield resin | 0.204 mmol | 200 mg |
| tert-butyl (S)-(1-(butylamino)-3-(4-hydroxyphenyl)-1-oxopropan-2-yl)carbamate 16 | 0.24 mmol | 100 mg |
| Potassium iodide | 0.02 mmol | 3.4 mg |
| Sodium hydride (60% in mineral oil) | 0.31 mmol | 12 mg |
| N,N-dimethylformamide | 0.051 M | 4 mL |
CRITICAL: Sodium hydride reacts violently with water, releasing flammable hydrogen gas, thus it is crucial to keep it away from moisture. Sodium hydride can ignite spontaneously in the presence of moisture or air, leading to a fire hazard. It can cause corrosion and burns upon direct contact with the skin, eyes, and mucous membranes.
Synthesis of the heterogeneous catalysts 22a – 22h
| Reagent | Concentration | Amount |
|---|---|---|
| (Aminomethylated)polystyrene | 0.6 mmol | 500 mg |
| Boc-(L)-methionine 20a | 0.72 mmol | 180 mg |
| Boc-O-benzyl-(L)-serine 20b | 0.72 mmol | 213 mg |
| Boc-(L)-2-propargylglycine 20c | 0.72 mmol | 154 mg |
| Boc-(L)-leucine 20d | 0.72 mmol | 167 mg |
| Boc-(L)-alanine 20e | 0.72 mmol | 136 mg |
| Boc-(L)-phenylalanine 20f | 0.72 mmol | 191 mg |
| Boc-(L)-α-phenylglycine 20g | 0.72 mmol | 181 mg |
| Boc-(L)-α-tert-butylglycine 20h | 0.72 mmol | 167 mg |
| Hydroxybenzotriazole | 0.78 mmol | 105 mg |
| N,N-diisopropylcarbodiimide | 0.72 mmol | 113 μL |
| 4-dimethylaminopyridine | 0.06 mmol | 7.3 mg |
| N,N-dimethylformamide | 0.15 M | 4 mL |
For end-capping with acetic anhydride
| Reagent | Concentration | Amount |
|---|---|---|
| Acetic anhydride | 1.2 mmol | 113 μL |
| Pyridine | 1.2 mmol | 97 μL |
For end-capping with methyl iodide
| Reagent | Concentration | Amount |
|---|---|---|
| Methyl iodide | 2.4 mmol | 149 μL |
| Potassium carbonate | 1.2 mmol | 166 mg |
For end-capping with pivaloyl chloride
| Reagent | Concentration | Amount |
|---|---|---|
| Pivaloyl chloride | 1.2 mmol | 147 μL |
| Triethylamine | 1.2 mmol | 167 μL |
CRITICAL: Hydroxybenzotriazole may cause skin and eye irritation, thus direct contact with the skin or eyes should be avoided. Heating or burning hydroxybenzotriazole may produce toxic fumes, and inhalation of these fumes should be avoided. Hydroxybenzotriazole is harmful to aquatic life, thus releasing it into water systems should be avoided. N,N-diisopropylcarbodiimide (DIC) is highly flammable, keep it away from open flames, sparks, and heat sources. It can cause irritation to the eyes, skin, and respiratory system, thus direct contact with the eyes, skin, or inhalation of vapors should be avoided. DIC is reactive and should be handled carefully to prevent unwanted reactions. It reacts violently with water, releasing toxic fumes of carbon dioxide. 4-Dimethylaminopyridine (DMAP) may cause irritation to the eyes, skin, and respiratory system, thus direct contact with the eyes, skin, or inhalation of vapors should be avoided. DMAP is toxic if ingested, thus ingestion should be avoided. Acetic anhydride is corrosive and can cause severe burns and irritation to the skin, eyes, and respiratory tract. Inhalation of vapors or ingestion can be harmful and may cause respiratory and gastrointestinal issues. It may cause irritation upon contact with the skin, eyes, and mucous membranes. Acetic anhydride is highly flammable and poses a fire hazard. It may form explosive mixtures in the air. It reacts violently with water, liberating acetic acid, thus care should be taken to avoid moisture. Pyridine is toxic and can cause harmful effects if swallowed, inhaled, or absorbed through the skin. It may cause irritation to the eyes, skin, and respiratory tract upon contact. Pyridine is highly flammable and poses a fire hazard. It reacts with strong oxidizing agents and can form explosive mixtures. Methyl iodide is highly toxic and can cause harmful effects if inhaled, ingested, or absorbed through the skin. It can cause burns and irritation to the skin, eyes, and mucous membranes upon direct contact. It is flammable and poses a fire hazard. Pivaloyl chloride is corrosive and can cause burns and irritation to the skin, eyes, and mucous membranes upon direct contact. It reacts violently with water and moisture, releasing hydrogen chloride gas. Pivaloyl chloride is flammable and poses a fire hazard.
Step-by-step method details
The deprotection processes for both Boc-protected and Fmoc-protected catalysts follow identical procedures. In this context, the initial exposition provides general step-by-step methods applicable to either one or another type of protected catalysts. To maintain textual clarity and prevent information overload, specific catalyst preparation procedures refer to these overarching methods where applicable.
Deprotection of the Boc-protected catalysts
Timing: 26 h
The section detailing the general procedure for the deprotection of Boc-protected catalysts serves as a comprehensive guide for the systematic removal of tert-butoxycarbonyl (Boc) protecting groups from heterogeneous catalysts. Boc protection is commonly employed in organic synthesis to shield amino groups and prevent undesired reactions. This deprotection step is crucial for revealing the active sites of the catalyst, ensuring its proper functionality in subsequent reactions. The outlined procedure provides a step-by-step protocol, including specific reagents and reaction conditions, allowing to effectively carry out the deprotection process (Scheme 1).
-
1.Boc cleavage.
-
a.Weigh 150 mg of Boc-protected catalyst and transfer it into a 4 mL vial. Troubleshooting 1.
-
b.Add 1.5 mL of dichloromethane (DCM) and seal the vial with a screw cover.
-
c.Attach the vial to the shaker.
-
d.Set-up shaker to the shaking speed 5 (approximately 220 rpm) and shake the vial for 20 min. Troubleshooting 2.
-
e.Remove as much DCM as possible using 1 mL pipette.Note: Mostly, beads form a layer either on the surface of the DCM or on the bottom of the vial. However, in some cases (depending on the catalyst’s properties), beads disperse evenly in the solvent, thus complicating removal of the solvent. If this problem occurs, let the vial stay for 5 min and remove as much solvent as possible. Alternatively, filtration can be used to overcome this problem.
-
f.Add 1.5 mL of 50% trifluoroacetic acid (TFA) solution in DCM (v/v) and seal the vial with a screw cover.
-
g.Attach the vial to the shaker.
-
h.Set-up shaker to the shaking speed 7 (approximately 340 rpm) and shake the vial for 5 min.
-
i.Remove as much DCM as possible using 1 mL pipette.
-
j.Repeat steps 1f – 1h, but this time shake the vial for 20 min. Troubleshooting 2.
-
k.Set-up the vacuum filtration system using 25 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
l.Transfer beads to the filter funnel using a 1 mL pipette.Note: Before transferring beads, ensure thorough resuspension to enhance collection of catalyst’s beads efficiency. In case of solvent depletion with a significant amount of catalyst remaining in the vial, introduce 0.5 mL of DCM, resuspend the reaction mixture using 1mL pipette, and subsequently transfer it to the filtration system. Repeat this procedure until the complete transfer of the catalyst to the filtration system is achieved.
-
m.Remove the solvent using vacuum.
-
n.Add 0.5 mL of fresh DCM to the filter funnel, then remove the solvent using vacuum.
-
o.Repeat step 1n alternating iPrOH (3 times) and DCM (3 times).
-
a.
-
2.Neutralization.
-
a.Transfer beads into another 4 mL vial. Troubleshooting 1.
-
b.Repeat steps 1f – 1o, but this time using 10% triethylamine solution in DCM (v/v) to generate free amine from the TFA salt. Troubleshooting 2.
-
a.
-
3.Drying the active deprotected catalyst.
-
a.Transfer deprotected catalyst to the 4 mL vial. Troubleshooting 1.
-
b.Dry under the high vacuum (0.1 mbar) at 20°C–22°C for 24 h.
-
a.
Note: Store deprotected catalysts at 0°C–4°C.
Scheme 1.
General scheme for the deprotection of the Boc-protected heterogeneous catalysts
Deprotection of the Fmoc-protected catalysts
Timing: 25 h 30 min
The section detailing the general procedure for the deprotection of Fmoc-protected catalysts serves as a comprehensive guide for the systematic removal of fluorenylmethyloxycarbonyl (Fmoc) protecting groups from heterogeneous catalysts. Fmoc protection is commonly employed in organic synthesis to shield amino groups and prevent undesired reactions. This deprotection step is crucial for revealing the active sites of the catalyst, ensuring its proper functionality in subsequent reactions. The outlined procedure provides a step-by-step protocol, including specific reagents and reaction conditions, allowing to effectively carry out the deprotection process (Scheme 2).
-
4.Fmoc cleavage.
-
a.Weigh 150 mg of Fmoc-protected catalyst and transfer it into a 4 mL vial. Troubleshooting 1.
-
b.Add 1.5 mL of N,N-dimethylformamide (DMF) and seal the vial with a screw cover.
-
c.Attach the vial to the shaker.
-
d.Set-up shaker to the shaking speed 5 (approximately 220 rpm) and shake the vial for 20 min. Troubleshooting 2.
-
e.Remove as much DMF as possible using 1 mL pipette.Note: Mostly, beads tend to form a layer either at the surface of the DMF or at the bottom of the vial. Nonetheless, in certain cases, depending on the properties of the catalyst, beads disperse uniformly throughout the solvent, thereby complicating solvent removal. If this issue arises, allow the vial to stand for 5 minutes and extract as much solvent as possible. Alternatively, filtration can be employed to overcome this complication.
-
f.Add 1.5 mL of 20% piperidine solution in DMF (v/v) and seal the vial with a screw cover.
-
g.Attach the vial to the shaker.
-
h.Set-up shaker to the shaking speed 7 (approximately 340 rpm) and shake the vial for 5 min.
-
i.Remove as much DMF as possible using 1 mL pipette.
-
j.Repeat steps 1f – 1h, but this time shake the vial for 10 min. Troubleshooting 2.
-
k.Set-up the vacuum filtration system using 25 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
l.Transfer beads to the filter funnel using a 1 mL pipette.Note: Before transferring beads, ensure thorough resuspension to enhance collection of catalyst’s beads efficiency. In case of solvent depletion with a significant amount of catalyst remaining in the vial, introduce 0.5 mL of DMF, resuspend the reaction mixture using 1 mL pipette, and subsequently transfer it to the filtration system. Repeat this procedure until the complete transfer of the catalyst to the filtration system is achieved.
-
m.Remove the solvent using vacuum.
-
n.Add 0.5 mL of fresh DMF to the filter funnel, then remove the solvent using vacuum.
-
o.Repeat step 1n alternating DMF (3 times) and iPrOH (3 times).
-
p.Repeat step 1n using THF (3 times), THF/MeOH (1:1 mixture) and then again THF (3 times).
 CRITICAL: Step 1p is extremely crucial, as the omission of this step will result in the persistence of DMF residue. Such residual presence not only contaminates the catalyst, affecting the reaction's outcome, but also artificially elevates the nitrogen ratio in the elemental analysis, consequently increasing the recorded catalyst loading.
CRITICAL: Step 1p is extremely crucial, as the omission of this step will result in the persistence of DMF residue. Such residual presence not only contaminates the catalyst, affecting the reaction's outcome, but also artificially elevates the nitrogen ratio in the elemental analysis, consequently increasing the recorded catalyst loading.
-
a.
-
5.Drying the active deprotected catalyst.
-
a.Transfer deprotected catalyst to the 4 mL vial. Troubleshooting 1.
-
b.Dry under the high vacuum (0.1 mbar) at 20°C–22°C for 24 h.
-
a.
CRITICAL: The efficiency of the deprotection step can be assessed through IR spectroscopy, wherein the disappearance of the characteristic Boc-carbonyl band is monitored. The loading of the resulting catalyst can be quantified based on the nitrogen content determined through elemental analysis.
Note: Store deprotected catalysts at 0°C–4°C.
Scheme 2.
General scheme for the deprotection of the Fmoc-protected heterogeneous catalysts
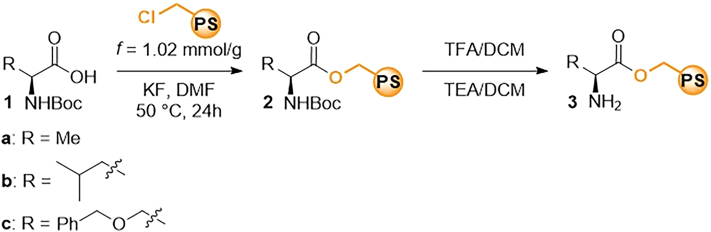
Synthesis of the heterogeneous catalysts 3a – 3c on the merrifield resin
Timing: for synthesis and storage in protected form – 2 days 1 h; for synthesis and deprotection – 2 days 3 h
The section outlining the general procedure for the synthesis of heterogeneous catalysts on Merrifield resin achieves a comprehensive description of the synthetic steps involved in covalently anchoring Boc-protected amino acids to the well-established Merrifield resin. The process involves nucleophilic displacement of chlorine atoms through the use of potassium fluoride, activating carboxylic acid moieties of amino acids. The result is the formation of catalysts intricately bonded to the Merrifield resin, characterized by loadings within the range of 0.59–0.64 mmol/g (Scheme 3).
-
6.Set-up the reaction.
-
a.Weigh 200 mg of Merrifield resin (f = 1.02 mmol/g) and transfer it into a 4 mL vial. Troubleshooting 1.
-
b.Add 1.5 mL of DMF and seal the vial with a screw cover.
-
c.Allow resin to swell at room temperature (20°C–22°C) for 20 min.
-
d.Weight a Boc-protected amino acid (1.5 eq) and add it to the suspension of resin in DMF.Note: Boc-protected amino acids: Boc-(L)-alanine 1a (58 mg), Boc-(L)-leucine 1b (71 mg), Boc-O-benzyl-(L)-serine 1c (90 mg).
-
e.Weight 36 mg of potassium fluoride (3 eq), add it to the suspension of resin in DMF and seal the vial with a screw cover.
-
f.Preheat a thermoregulated shaker to 50°C.
-
g.Put the vial into the shaker.Note: The appropriate rack for corresponding size of the vial was attached to the shaker. The alternative attachment methods are acceptable as long as the vial remains stable during the reaction.
-
h.Set-up shaker to the shaking speed 5 (approximately 265 rpm).
-
i.Let the reaction proceed for 24 h. Troubleshooting 2.
-
a.
-
7.Purify the crude Boc-protected catalysts 2a – 2c.
-
a.Set-up the vacuum filtration system using 100 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
b.Transfer the reaction mixture to the filter funnel using a 1 mL pipette.Note: Before transferring the reaction mixture, ensure thorough resuspension to enhance collection of catalyst’s beads efficiency. In case of solvent depletion with a significant amount of catalyst remaining in the vial, introduce 0.5 mL of DMF, resuspend the reaction mixture using 1 mL pipette, and subsequently transfer it to the filtration system. Repeat this procedure until the complete transfer of the catalyst to the filtration system is achieved.
-
c.Remove the solvent using vacuum.
-
d.Add 3 mL of fresh DMF to the filter funnel, then remove the solvent using vacuum.
-
e.Repeat step 2d.
-
f.Repeat step 2d using instead of DMF successively DMF/H2O (1:1 mixture, 3 times), MeOH/H2O (1:1 mixture, 3 times) and then MeOH (3 times).
-
a.
-
8.Dry the purified Boc-protected catalysts 2a – 2c.
-
a.Transfer purified catalyst to the 4 mL vial. Troubleshooting 1.
-
b.Dry under the high vacuum (0.1 mbar) at 20°C–22°C for 24 h.
-
a.
CRITICAL: The efficiency of the implementation of the amino acids to the resin can be assessed through IR spectroscopy, wherein the appearance of the characteristic Boc-carbonyl band is monitored.
-
9.
Step 3 can be omitted if immediate use of the freshly deprotected catalyst is required. However, for prolonged storage, it is advisable to maintain the catalysts in their protected form. Deprotect the Boc-protected catalysts 2a–2c following “Deprotection of the Boc-protected catalysts” procedure to obtained corresponding catalysts 3a–3c.
Scheme 3.
General synthetic scheme for the preparation of the heterogeneous catalysts on the Merrifield resin
Synthesis of the heterogeneous catalysts 6a – 6d on the wang resin
Timing: for synthesis and storage in protected form – 29 h; for synthesis and deprotection – 30 h 30 min
The section detailing the general procedure for the synthesis of heterogeneous catalysts on the Wang resin accomplishes a comprehensive guide for the functionalization of the Wang resin with Fmoc-protected amino acids. This synthetic process involves the application of the classic peptide coupling system, utilizing hydroxybenzotriazole (HOBt) and N,N′-diisopropylcarbodiimide (DIC). To ensure the elimination of potential interference from residual free hydroxy groups on the resin, an additional blocking step with acetic anhydride is incorporated. The result is the generation of catalysts with loadings ranging from 0.38 to 0.69 mmol/g (Scheme 4).
-
10.Set-up the reaction.
-
a.Weigh 200 mg of Wang resin (f = 1.2 mmol/g) and transfer it into a 10 mL vial. Troubleshooting 1.
-
b.Add 2.7 mL of DCM and 0.3 mL of DMF and seal the vial with a screw cover.
-
c.Allow resin to swell at room temperature (20°C–22°C) for 20 min.
-
d.Weight Fmoc-protected amino acid (2 eq) and add it to the suspension of resin in DCM/DMF mixture.Note: Boc-protected amino acids: 186 mg of Fmoc-(L)-phenylalanine 4a, 170 mg of Fmoc-(L)-isoleucine 4b, 163 mg of Fmoc-(L)-valine 4c, 149 mg of Fmoc-(L)-alanine 4d.
-
e.Weight 65 mg of hydroxybenzotriazole (2 eq), 38 μL of N,N-diisopropylcarbodiimide (1 eq), 3 mg of 4-dimethylaminopyridine (0.1 eq), add these to the suspension of resin in DCM/DMF mixture and seal the vial with a screw cover.
-
f.Attach the vial to the shaker.
-
g.Set-up shaker to the shaking speed 7 (approximately 265 rpm).
-
h.Let the reaction proceed for 3 h at room temperature (20°C–22°C). Troubleshooting 2.
-
a.
-
11.End-cap remaining unreacted hydroxyl groups on the resin.
-
a.Weight 45 μL of acetic anhydride (2 eq), 39 μL of pyridine (2 eq), add these to the suspension of crude Fmoc-protected catalysts in DCM/DMF mixture and seal the vial with a screw cover.
-
b.Attach the vial to the shaker.
-
c.Set-up shaker to the shaking speed 7 (approximately 355 rpm).
-
d.Let the reaction proceed for 30 min at room temperature (20°C–22°C). Troubleshooting 2.
-
a.
-
12.Purify the crude end-capped Boc-protected catalysts 5a – 5d.
-
a.Set-up the vacuum filtration system using 100 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
b.Transfer the reaction mixture to the filter funnel using a 1 mL pipette.Note: Before transferring the reaction mixture, ensure thorough resuspension to enhance collection of catalyst’s beads efficiency. In case of solvent depletion with a significant amount of catalyst remaining in the vial, introduce 0.5 mL of DCM or DMF, resuspend the reaction mixture using 1 mL pipette, and subsequently transfer it to the filtration system. Repeat this procedure until the complete transfer of the catalyst to the filtration system is achieved.
-
c.Remove the solvent using vacuum.
-
d.Add 3 mL of fresh DMF to the filter funnel, then remove the solvent using vacuum.
-
e.Repeat step 3d.
-
f.Repeat step 3d using instead of DMF successively DCM (3 times) and MeOH (3 times).
-
a.
-
13.Dry the purified end-capped Boc-protected catalysts 5a–5d.
-
a.Transfer purified catalyst to the 4 mL vial. Troubleshooting 1.
-
b.Dry under the high vacuum (0.1 mbar) at 20°C–22°C for 24 h.
-
a.
CRITICAL: The efficiency of the implementation of the amino acids to the resin can be assessed through IR spectroscopy, wherein the appearance of the characteristic Boc-carbonyl band is monitored.
-
14.
Step 4 can be omitted if immediate use of the freshly deprotected catalyst is required. However, for prolonged storage, it is advisable to maintain the catalysts in their protected form. Deprotect the Fmoc-protected catalysts 5a–5d following “Deprotection of the Fmoc-protected catalysts” procedure to obtained corresponding catalysts 6a–6d.
Scheme 4.
General synthetic scheme for the preparation of the heterogeneous catalysts on the Wang resin
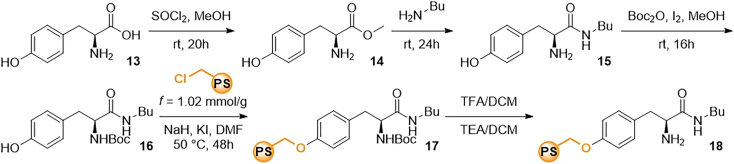
Synthesis of the heterogeneous catalyst 12
Timing: 30 h (for step 16)
Timing: 10 h (for step 17)
Timing: for synthesis and storage in protected form – 3 days 2 h; for synthesis and deprotection – 3 days 4 h (for step 18)
The section detailing the full procedure for the synthesis of the heterogeneous catalyst derived from a quinine derivative on Merrifield resin accomplishes a comprehensive guide for the multi-step process involved in functionalizing the resin. The result is a catalyst with a functionalization level of 0.6 mmol/g. This section not only provides the step-by-step protocol for the synthesis of the heterogeneous catalyst but also includes detailed procedures for the preparation of the corresponding starting material containing free hydroxy group crucial for the immobilization phase (Scheme 5).
-
15.
Prepare the (S)-(6-methoxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methanamine 8 starting from quinine 7 following a published procedure.8 Troubleshooting 3.
-
16.Prepare the 4-((S)-amino((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)quinolin-6-ol 9.
-
a.Weigh 355 mg of (S)-(6-methoxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methanamine 8 (1.1 mmol) to the two-neck 25 mL round-bottom flask.
-
b.Add 4 mL of DCM and magnetic stirring bar, then seal the flack with two rubber septa.
-
c.Prepare a cooling bath at −78°C.
-
i.Scoop dry ice into the suitable container (glassware, insulated dewar or Teflon bath) so that it fills approximately half of the container.
-
ii.Attach a low-temperature thermometer.
-
iii.Using a squirt bottle, carefully add acetone to the dry ice to produce a slurry. Confirm that the slurry reaches −78°C.
 CRITICAL: Add acetone slowly, as the large volumes of carbon dioxide produced will cause rapid bubbling, leading to the potential splashing out of acetone from the bath.Note: If the temperature does not reach −78°C, add more dry ice. If the slurry obtained is too thick, add more acetone.
CRITICAL: Add acetone slowly, as the large volumes of carbon dioxide produced will cause rapid bubbling, leading to the potential splashing out of acetone from the bath.Note: If the temperature does not reach −78°C, add more dry ice. If the slurry obtained is too thick, add more acetone.
-
i.
-
d.Attach the reaction flask to the metal bars in the hood and lower it into the prepared cooling bath so that the level of the solution in the flask fully immerses into the acetone/dry ice mixture.
-
e.Draw 5.5 mL of boron tribromide (5.49 mmol, 1 M solution in DCM) with 10 mL plastic syringe and add it to the reaction mixture dropwise under the argon atmosphere.Note: To fill the syringe with the boron tribromide solution in DCM, introduce an argon flow through the bottle using two needles. Aim to fill the syringe expeditiously and promptly transfer it to the reaction flask via septum, considering that the boron tribromide solution in DCM tends to drip from the syringe.
-
f.Take the reaction flask out from the cooling bath and allow it to warm to room temperature (20°C–22°C).
-
g.Stir the reaction mixture for 24 h at room temperature (20°C–22°C).
-
h.Perform extraction of the resulting reaction mixture. Troubleshooting 4.
-
i.Add 15 mL of 1 M aqueous NaOH solution at 0°C.
-
ii.Transfer the quenched reaction mixture to a 100 mL separatory funnel.
-
iii.Wash the flask with 2 mL DCM three times into the separatory funnel, seal it with a stopper.
-
iv.Shake the funnel vigorously, and then keep it quiescent until the organic phase separates from the aqueous phase.
-
v.Pour the organic phase into a 100 mL Erlenmeyer flask.
-
vi.Repeat the extraction with 10 mL DCM two times.
-
vii.Add 6 M aqueous HCl to the aqueous phase until pH 7 is reached.
-
viii.Add 10 mL of DCM into the separatory funnel containing neutralized aqueous phase, seal it with a stopper.
-
ix.Shake the funnel vigorously, and then keep it quiescent until the organic phase separates from the aqueous phase.
-
x.Pour the organic phase into a 250 mL Erlenmeyer flask.
-
xi.Repeat the extraction with 10 mL DCM nine times.Note: Check the efficiency of the extraction using thin layer chromatography (TLC). TLC conditions: DCM (1.9 mL)/MeOH (NH3) (0.1 mL).
-
i.
-
i.Dry the combined organic phase.
-
i.Add 50 g of anhydrous sodium sulfate (Na2SO4) into the Erlenmeyer flask.
-
ii.Add magnetic stirring bar and stir for 2 h at room temperature (20°C–22°C).
-
i.
-
j.Filter obtained crude product.
-
i.Set-up vacuum filtration system using 500 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
ii.Pour the extracted reaction mixture into the filter funnel.
-
iii.Remove the solvent using vacuum.
-
iv.Wash the Erlenmeyer flask twice with 10 mL DCM pouring it into the filter funnel and remove the solvent using vacuum.
-
v.Repeat the process until the desired product is no longer detected, verifying its absence through TLC (TLC conditions: DCM (1.9 mL)/MeOH (NH3) (0.1 mL)).
-
i.
-
k.Remove the solvent using rotary evaporator at 40°C.
-
l.Transfer the crude product to a 25 mL round-bottom flask rinsing 500 mL round-bottom flask with DCM, remove the solvent using rotary evaporator at 40°C.
-
m.Finally remove the remaining solvent under high vacuum (0.1 mbar) at 20°C–22°C for 2 h to obtain the desired product 4-((S)-amino((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)quinolin-6-ol 9 that can be used in the next step without further purification.
-
a.
-
17.Prepare the tert-butyl ((S)-(6-hydroxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)carbamate 10.
-
a.Add 1.3 mL of MeOH to the 200 mg of 4-((S)-amino((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)ecarbona-6-ol 9 (0.65 mmol) obtained in the previous step 2m.
-
b.Add 212 mg of di-tert-butyl ecarbonate (0.97 mmol), 16.4 mg of iodine (0.065 mmol) and magnetic stirring bar, then seal the flack with stopper.
-
c.Stir the reaction mixture for 3 h at room temperature (20°C–22°C).
-
d.Perform extraction of the resulting reaction mixture. Troubleshooting 4.
-
i.Add 15 mL of saturated aqueous Na2S2O3 solution.
-
ii.Transfer the quenched reaction mixture to a 50 mL separatory funnel.
-
iii.Wash the flask with 2 mL Et2O three times into the separatory funnel, seal it with a stopper.
-
iv.Shake the funnel vigorously, and then keep it quiescent until the organic phase separates from the aqueous phase.
-
v.Separate the water phase from organic phase.
-
vi.Add 10 mL of saturated aqueous NaHCO3 solution to the organic phase. Repeat the extraction process.
-
vii.Pour the organic phase into a 25 mL Erlenmeyer flask.
-
i.
-
e.Dry combined organic phase.
-
i.Add 5 g of anhydrous Na2SO4 into the Erlenmeyer flask.
-
ii.Add magnetic stirring bar and stir for 2 h at room temperature (20°C–22°C).
-
i.
-
f.Filter the crude product.
-
i.Set-up vacuum filtration system using 50 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
ii.Pour the extracted reaction mixture into the filter funnel.
-
iii.Remove the solvent using vacuum.
-
iv.Wash the Erlenmeyer flask twice with 2 mL Et2O pouring it into the filter funnel and remove the solvent using vacuum.
-
v.Repeat the process until the desired product is no longer detected, verifying its absence through TLC (TLC conditions: DCM (1.8 mL)/MeOH (0.2 mL)).
-
i.
-
g.Remove the solvent using rotary evaporator at 40°C.
-
h.Purify the crude product (around 300 mg) using automated column chromatography.
-
i.Prepare a silica gel column using 20 g of silica gel.
-
ii.Pack the column with 3% of MeOH in DCM as eluent.
-
iii.Dissolve the crude product in a minimum amount of DCM.
-
iv.Transfer the solution to the silica gel column.
-
v.Wash the flask twice with a minimum amount of DCM and transfer to the silica gel column.
-
vi.Run the column chromatography with gradient of MeOH in DCM as eluent (1 column volume (CV) with 3% of MeOH, 13 CV with gradient of MeOH from 3% to 5%, 2CV with 5% of MeOH).
-
vii.Collect the sample with 20 mL test tubes.
-
viii.Confirm the presence of the desired product using TLC (TLC conditions: DCM (1.8 mL)/MeOH (0.2 mL)).
-
ix.Combine fractions containing the desired product into a 500 mL round-bottom flask.
-
i.
-
i.Remove the solvent using rotary evaporator at 40°C.
-
j.Transfer the purified product to a 10 mL round-bottom flask rinsing 500 mL round-bottom flask with DCM, remove the solvent using rotary evaporator.
-
k.Finally remove the remaining solvent under high vacuum (0.1 mbar) at 20°C–22°C for 2 h to obtain the desired product tert-butyl ((S)-(6-hydroxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)carbamate 10 as an amorphous off-white solid (181 mg, 68%).
-
a.
-
18.Prepare the immobilized catalyst 11.
-
a.Set-up the reaction.
-
i.Weigh 200 mg of Merrifield resin (f = 1.02 mmol/g) and 3.4 mg of potassium iodide (0.02 mmol), transfer these into a 10 mL vial. Troubleshooting 1.
-
ii.Add 4 mL of DMF and seal the vial with a screw cover.
-
iii.Allow resin to swell at room temperature (20°C–22°C) for 20 min.
-
iv.Weight 100 mg of tert-butyl ((S)-(6-hydroxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)carbamate 10 (0.24 mmol) and 12 mg of sodium hydride (0.31 mmol, 60% in mineral oil), add these to the suspension of resin in DMF and seal the vial with a screw cover.
-
v.Preheat a thermoregulated shaker to 50°C.
-
vi.Attach the vial to the shaker.
-
vii.Set-up shaker to the shaking speed 5 (approximately 265 rpm).
-
viii.Let the reaction proceed for 48 h. Troubleshooting 2.
-
i.
-
b.Purify the crude Boc-protected catalyst 11.
-
i.Set-up the vacuum filtration system using 100 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
ii.Transfer the reaction mixture to the filter funnel using a 1 mL pipette.Note: Before transferring the reaction mixture, ensure thorough resuspension to enhance collection of catalyst’s beads efficiency. In case of solvent depletion with a significant amount of catalyst remaining in the vial, introduce 0.5 mL of DMF, resuspend the reaction mixture using 1 mL pipette, and subsequently transfer it to the filtration system. Repeat this procedure until the complete transfer of the catalyst to the filtration system is achieved.
-
iii.Remove the solvent using vacuum.
-
iv.Add 5 mL of fresh DMF to the filter funnel, then remove the solvent using vacuum.
-
v.Repeat step 4biv.
-
vi.Repeat step 4biv using instead of DMF successively H2O (3 times), MeOH (3 times), MeOH/THF (1:1 mixture) (3 times) and THF (3 times).
-
i.
-
c.Dry the purified Boc-protected catalyst 11.
-
i.Transfer purified catalyst to the 4 mL vial. Troubleshooting 1.
-
ii.Dry under the high vacuum (0.1 mbar) at 20°C–22°C for 24 h.
 CRITICAL: The efficiency of the implementation of the amino acids to the resin can be assessed through IR spectroscopy, wherein the appearance of the characteristic Boc-carbonyl band is monitored.
CRITICAL: The efficiency of the implementation of the amino acids to the resin can be assessed through IR spectroscopy, wherein the appearance of the characteristic Boc-carbonyl band is monitored.
-
i.
-
d.Step 4c can be omitted if immediate use of the freshly deprotected catalyst is required. However, for prolonged storage, it is advisable to maintain the catalysts in their protected form. Deprotect the Boc-protected catalyst 11 following “Deprotection of the Boc-protected catalysts” procedure to obtain catalyst 12.
-
a.
Scheme 5.
Synthetic scheme for the preparation of the immobilized catalyst 12
See Cassani et al.8
Synthesis of the heterogeneous catalyst 18
Timing: 23 h (for step 20)
Timing: for synthesis and storage in protected form – 3 days 1 h; for synthesis and deprotection – 3 days 3 h (for step 21)
The section outlining the full procedure for the synthesis of the heterogeneous catalyst derived from a tyrosine accomplishes a detailed and systematic guide for the immobilization of corresponding tyrosine derivative to the Merrifield resin. The presence of a free hydroxy group allows for the anchoring of modified tyrosine, resulting in a catalyst with a loading of 0.34 mmol/g. Moreover, the procedure includes the description of steps involving the transformation of carboxylic acid to amide, a modification deemed necessary for the catalytic reactions studied in the main article (Scheme 6).
-
19.
Prepare the (S)-2-amino-N-butyl-3-(4-hydroxyphenyl)propenamide 15 through intermediate 14, starting from (L)-phenylalanine 13, following a published procedure.9
Note: Obtained crude (S)-2-amino-N-butyl-3-(4-hydroxyphenyl)propenamide 15 can be used for the next step without further purification.
-
20.Prepare the tert-butyl (S)-(1-(butylamino)-3-(4-hydroxyphenyl)-1-oxopropan-2-yl)carbamate 16.
-
a.Weigh 415 mg of (S)-2-amino-N-butyl-3-(4-hydroxyphenyl)propenamide 15 (1.76 mmol) to the 25 mL round-bottom flask.
-
b.Add 3.51 mL of MeOH and magnetic stirring bar.
-
c.Add 575 mg of di-tert-butyl dicarbonate (2.63 mmol) and 45 mg of iodine (0.18 mmol), seal the flack with stopper.
-
d.Stir the reaction mixture for 16 h at room temperature (20°C–22°C).
-
e.Perform extraction of the resulting reaction mixture. Troubleshooting 4.
-
i.Add 10 mL of saturated aqueous Na2S2O3 solution.
-
ii.Transfer the quenched reaction mixture to a 50 mL separatory funnel.
-
iii.Wash the flask with 2 mL Et2O three times into the separatory funnel, seal it with a stopper.
-
iv.Shake the funnel vigorously, and then keep it quiescent until the organic phase separates from the aqueous phase.
-
v.Separate the water phase from organic phase.
-
vi.Add 10 mL of saturated aqueous NaHCO3 solution to the organic phase. Repeat the extraction process following steps 2eii – 2ev.
-
vii.Pour the organic phase into a 25 mL Erlenmeyer flask.
-
i.
-
f.Dry combined organic phase.
-
i.Add 5 g of anhydrous Na2SO4 into the Erlenmeyer flask.
-
ii.Add magnetic stirring bar and stir for 2 h at room temperature (20°C–22°C).
-
i.
-
g.Filter the crude product.
-
i.Set-up vacuum filtration system using 50 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
ii.Pour the extracted reaction mixture into the filter funnel.
-
iii.Remove the solvent using vacuum.
-
iv.Wash the Erlenmeyer flask twice with 2 mL Et2O pouring it into the filter funnel and remove the solvent using vacuum.
-
v.Repeat the process until the desired product is no longer detected, verifying its absence through TLC (TLC conditions: DCM (1.8 mL)/MeOH (0.2 mL)).
-
i.
-
h.Remove the solvent using rotary evaporator at 40°C.
-
i.Purify the crude product (around 500 mg) using automated column chromatography.
-
i.Prepare a silica gel column using 40 g of silica gel.
-
ii.Pack the column with 3% of MeOH in DCM as eluent.
-
iii.Dissolve the crude product in a minimum amount of DCM.
-
iv.Transfer the solution to the silica gel column.
-
v.Wash the flask twice with a minimum amount of DCM and transfer to the silica gel column.
-
vi.Run the column chromatography with gradient of MeOH in DCM as eluent (1 column volume (CV) with 3% of MeOH, 4 CV with gradient of MeOH from 3% to 4%).
-
vii.Collect the sample with 20 mL test tubes.
-
viii.Confirm the presence of the desired product using TLC (TLC conditions: DCM (1.8 mL)/MeOH (0.2 mL)).
-
ix.Combine fractions containing the desired product into a 250 mL round-bottom flask.
-
i.
-
j.Remove the solvent using rotary evaporator at 40°C.
-
k.Transfer the purified product to a 10 mL round-bottom flask rinsing 250 mL round-bottom flask with DCM, remove the solvent using rotary evaporator at 40°C.
-
l.Finally remove the remaining solvent under high vacuum (0.1 mbar) at 20°C–22°C for 2 h to obtain the desired product tert-butyl (S)-(1-(butylamino)-3-(4-hydroxyphenyl)-1-oxopropan-2-yl)carbamate 16 as an off-white solid (528 mg, 89%).
-
a.
-
21.
Prepare the immobilized catalyst 18 following “Synthesis of the immobilized catalyst 12” procedure steps 4a – 4d.
Note: 82 mg of tert-butyl (S)-(1-(butylamino)-3-(4-hydroxyphenyl)-1-oxopropan-2-yl)carbamate 16 (0.24 mmol).
Scheme 6.
Synthetic scheme for the preparation of the immobilized catalyst 18
See Ranjbar et al.9
Synthesis of the heterogeneous catalysts 22a – 22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride
Timing: for synthesis and storage in protected form – 3 days 5 h; for synthesis and deprotection – 3 days 7 h
The section outlining the general procedure for the synthesis of heterogeneous catalysts on (aminomethylated)polystyrene achieves a detailed guide for introducing Boc-protected amino acids to the (aminomethylated)polystyrene resin. The utilization of the same coupling system allows for the exploration of the impact of the attachment bond between the catalytic moiety and the resin. Three distinct strategies are employed to block the remaining free amino groups of the resin, a crucial step to prevent unwanted background reactions in aminocatalysis. This section presents a variety of immobilized catalysts, end-capped with acetic and pivaloyl moieties or alkylated with methyl iodide. The resulting catalysts exhibit comparable functionalization levels, falling within the range of 0.38–0.59 mmol/g. This comprehensive procedure offers valuable insights into customizing (aminomethylated)polystyrene-supported catalysts for diverse applications. It emphasizes the significance of the end-capping strategy in ensuring enhanced reusability and facilitating efficient recovery after reactions (Scheme 7).
-
22.Set-up the reaction.
-
a.Weigh 500 mg of (aminomethylated)polystyrene (f = 1.2 mmol/g) and transfer it into a 10 mL vial. Troubleshooting 1.
-
b.Add 4 mL of DMF and seal the vial with a screw cover.
-
c.Allow resin to swell at room temperature (20°C–22°C) for 20 min.
-
d.Weight a Boc-protected amino acid (1.2 eq) and add it to the suspension of resin in DMF.Note: Boc-protected amino acids: Boc-(L)-methionine 20a (180 mg), Boc-O-benzyl-(L)-serine 20b (213 mg), Boc-(L)-2-propargylglycine 20c (154 mg), Boc-(L)-leucine 20d (167 mg), Boc-(L)-alanine 20e (136 mg), Boc-(L)-phenylalanine 20f (191 mg), Boc-(L)-α-phenylglycine 20g (181 mg), Boc-(L)-α-tert-butylglycine 20h (167 mg).
-
e.Weight 105 mg of hydroxybenzotriazole (1.3 eq), 113 μL of N,N-diisopropylcarbodiimide (1.2 eq), 7.3 mg of 4-dimethylaminopyridine (0.1 eq), add these to the suspension of resin in DMF and seal the vial with a screw cover.
-
f.Preheat a thermoregulated shaker to 60°C.
-
g.Put the vial into the shaker.Note: The appropriate rack for corresponding size of the vial was attached to the shaker. The alternative attachment methods are acceptable as long as the vial remains stable during the reaction.
-
h.Set-up shaker to the shaking speed 5 (approximately 265 rpm).
-
i.Let the reaction proceed for 48 h. Troubleshooting 2.
-
a.
-
23.Wash the crude Boc-protected catalysts 21.
-
a.Set-up the vacuum filtration system using 50 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
b.Transfer the reaction mixture to the filter funnel using a 1 mL pipette.Note: Before transferring the reaction mixture, ensure thorough resuspension to enhance collection of catalyst’s beads efficiency. In case of solvent depletion with a significant amount of catalyst remaining in the vial, introduce 0.5 mL of DMF, resuspend the reaction mixture using 1 mL pipette, and subsequently transfer it to the filtration system. Repeat this procedure until the complete transfer of the catalyst to the filtration system is achieved.
-
c.Remove the solvent using vacuum.
-
d.Add 5 mL of fresh DMF to the filter funnel, then remove the solvent using vacuum.
-
e.Repeat step 2m (3 times).
-
a.
-
24.End-cap remaining unreacted amino groups on the resin.
-
a.Transfer beads into another 10 mL vial. Troubleshooting 1.
-
b.Add 4 mL of DMF.
-
c.Add reagent 1 and reagent 2 to the suspension of crude Boc-protected catalyst in DMF and seal the vial with a screw cover.
 CRITICAL: To prepare catalysts 22a’–22e′ use: reagent 1 – acetic anhydride (2 eq, 113 μL), reagent 2 – pyridine (2 eq, 97 μL). To prepare the catalyst 22e’’ use: reagent 1 – methyl iodide (4 eq, 149 μL), reagent 2 – potassium carbonate (2 eq, 166 mg). To prepare catalysts 22e’’’–22h’’’ use: reagent 1 – pivaloyl chloride (2 eq, 147 μL), reagent 2 – triethylamine (2 eq, 167 μL).
CRITICAL: To prepare catalysts 22a’–22e′ use: reagent 1 – acetic anhydride (2 eq, 113 μL), reagent 2 – pyridine (2 eq, 97 μL). To prepare the catalyst 22e’’ use: reagent 1 – methyl iodide (4 eq, 149 μL), reagent 2 – potassium carbonate (2 eq, 166 mg). To prepare catalysts 22e’’’–22h’’’ use: reagent 1 – pivaloyl chloride (2 eq, 147 μL), reagent 2 – triethylamine (2 eq, 167 μL). -
d.Preheat a shaker to 60°C.
-
e.Put the vial into the shaker.Note: The appropriate rack for corresponding size of the vial was attached to the shaker. The alternative attachment methods are acceptable as long as the vial remains stable during the reaction.
-
f.Set-up shaker to the shaking speed 5 (approximately 265 rpm).Note: Different shaking speeds were also tested. No changes in the outcomes were observed at shaking speed in the range from 150 rpm to 500 rpm.
-
g.Let the reaction proceed for 3 h. Troubleshooting 2.
-
a.
-
25.Purify the crude end-capped Boc-protected catalysts.
-
a.Set-up the vacuum filtration system using 100 mL round-bottom flask, vacuum filtration adapter, rubber conical gasket and filter funnel (porosity P3 (pore size 16–40 μm) or P4 (pore size 10–16 μm)).
-
b.Transfer the reaction mixture to the filter funnel using a 1 mL pipette.Note: Before transferring the reaction mixture, ensure thorough resuspension to enhance collection of catalyst’s beads efficiency. In case of solvent depletion with a significant amount of catalyst remaining in the vial, introduce 0.5 mL of DMF, resuspend the reaction mixture using 1 mL pipette, and subsequently transfer it to the filtration system. Repeat this procedure until the complete transfer of the catalyst to the filtration system is achieved.
-
c.Remove the solvent using vacuum.
-
d.Add 4 mL of fresh DMF to the filter funnel, then remove the solvent using vacuum.
-
e.Repeat step 4r.
-
f.Repeat step 4r using instead of DMF successively H2O (3 times), MeOH (3 times), MeOH/THF (1:1 mixture, 3 times) and then THF (3 times).
-
a.
-
26.Dry the purified end-capped Boc-protected catalyst.
-
a.Transfer purified catalyst to the 4 mL vial. Troubleshooting 1.
-
b.Dry under the high vacuum (0.1 mbar) at 20°C–22°C for 24 h.
-
a.
CRITICAL: The efficiency of the implementation of the amino acids to the resin can be assessed through IR spectroscopy, wherein the appearance of the characteristic Boc-carbonyl band is monitored.
-
27.
Step 5 can be omitted if immediate use of the freshly deprotected catalyst is required. However, for prolonged storage, it is advisable to maintain the catalysts in their protected form. Deprotect the Boc-protected catalyst 21 following “Deprotection of the Boc-protected catalysts” procedure.
Scheme 7.
General synthetic scheme for the preparation of the heterogeneous catalysts on the (aminomethylated)polystyrene
Expected outcomes
Catalyst 3a was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 3a–3c on the Merrifield resin” starting from Boc-(L)-alanine 1a (Scheme 3).
Catalyst 3b was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 3a–3c on the Merrifield resin” starting from Boc-(L)-leucine 1b (Scheme 3).
Catalyst 3c was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 3a–3c on the Merrifield resin” starting from Boc-O-benzyl-(L)-serine 1c (Scheme 3).
Catalyst 6a was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 6a–6d on the Wang resin” starting from Fmoc-(L)-phenylalanine 4a (Scheme 4).
Catalyst 6b was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 6a–6d on the Wang resin” starting from Fmoc-(L)-isoleucine 4b (Scheme 4).
Catalyst 6c was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 6a–6d on the Wang resin” starting from Fmoc-(L)-valine 4c (Scheme 4).
Catalyst 6d was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 6a–6d on the Wang resin” starting from Fmoc-(L)-alanine 4d (Scheme 4).
Catalyst 12 was prepared according to the multiple-step procedure “Synthesis of the immobilized catalyst 12” starting from quinine 7 through intermediates (S)-(6-methoxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methanamine 8, 4-((S)-amino((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)quinolin-6-ol 9 and tert-butyl ((S)-(6-hydroxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)carbamate 10 (Scheme 5).
Catalyst 18 was prepared according to the multiple-step procedure “Synthesis of the immobilized catalyst 18” starting from (L)-phenylalanine 13 through intermediates methyl L-tyrosinate 14, (S)-2-amino-N-butyl-3-(4-hydroxyphenyl)propenamide 15, tert-butyl (S)-(1-(butylamino)-3-(4-hydroxyphenyl)-1-oxopropan-2-yl)carbamate 16 (Scheme 6).
Catalyst 22a′ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-(L)-methionine 20a. End-capping was performed using acetic anhydride (Scheme 7).
Catalyst 22b′ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-O-benzyl-(L)-serine 20b. End-capping was performed using acetic anhydride (Scheme 7).
Catalyst 22c′ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-(L)-2-propargylglycine 20c. End-capping was performed using acetic anhydride (Scheme 7).
Catalyst 22d′ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-(L)-leucine 20d. End-capping was performed using acetic anhydride (Scheme 7).
Catalyst 22e′ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-(L)-alanine 20e. End-capping was performed using acetic anhydride (Scheme 7).
Catalyst 22e’’ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-(L)-alanine 20e. End-capping was performed using methyl iodide (Scheme 7).
Catalyst 22e’’’ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-(L)-alanine 20e. End-capping was performed using pivaloyl chloride (Scheme 7).
Catalyst 22f’’’ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-(L)-phenylalanine 20f. End-capping was performed using pivaloyl chloride (Scheme 7).
Catalyst 22g’’’ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-(L)-α-phenylglycine 20g. End-capping was performed using pivaloyl chloride (Scheme 7).
Catalyst 22h’’’ was prepared according to the general procedure “Synthesis of the heterogeneous catalysts 22a–22h on the (aminomethylated)polystyrene end-capped with acetic anhydride, methyl iodine or pivaloyl chloride” starting from Boc-(L)-α-tert-butylglycine 20h. End-capping was performed using pivaloyl chloride (Scheme 7).
Quantification and statistical analysis
IR spectroscopy parameters: resolution 4 cm−1, number of scans 24. For the performance of the IR spectroscopy see Troubleshooting 5.
Calculation of the functionalization level (loading) based on the nitrogen elemental analysis:
f (mmol/g) = percentage N · 1000 · (number of N atoms)−1 MW−1 · 100−1.
Figure 1.
Images of the deprotected immobilized catalysts 3a (A), 6a (B), 12 (C), 18 (D) and 22e’’’ (E)
Figure 2.
SEM images of deprotected isolated catalysts 6a (A) and 22e’’’ (B)
Catalyst 3a: IR (KBr): ν 1732 cm-1 (carbonyl band); 1716 cm-1 (Boc-protecting group carbonyl band of protected catalyst 2a), N elemental analysis (percentage): 0.9, f = 0.64 mmol/g. Image of the deprotected catalyst 3a is presented in Figure 1A.
Catalyst 3b: IR (KBr): ν 1731 cm-1 (carbonyl band); 1718 cm-1 (Boc-protecting group carbonyl band of protected catalyst 2b), N elemental analysis (percentage): 0.82, f = 0.59 mmol/g.
Catalyst 3c: IR (KBr): ν 1736 cm-1 (carbonyl band); 1716 cm-1 (Boc-protecting group carbonyl band of protected catalyst 2c), N elemental analysis (percentage): 0.85, f = 0.61 mmol/g.
Catalyst 6a: IR (KBr): ν 1732 cm-1 (carbonyl band); 1716 cm-1 (Fmoc-protecting group carbonyl band of protected catalyst 5a), N elemental analysis (percentage): 0.69, f = 0.49 mmol/g. Image of the deprotected catalyst 6a is presented in Figure 1B and SEM image is presented in Figure 2A.
Catalyst 6b: IR (KBr): ν 1732 cm-1 (carbonyl band); 1718 cm-1 (Fmoc-protecting group carbonyl band of protected catalyst 5b), N elemental analysis (percentage): 0.38, f = 0.27 mmol/g.
Catalyst 6c: IR (KBr): ν 1732 cm-1 (carbonyl band); 1719 cm-1 (Fmoc-protecting group carbonyl band of protected catalyst 5c), N elemental analysis (percentage): 0.42, f = 0.3 mmol/g.
Catalyst 6d: IR (KBr): ν 1732 cm-1 (carbonyl band); 1716 cm-1 (Fmoc-protecting group carbonyl band of protected catalyst 5d), N elemental analysis (percentage): 0.63, f = 0.45 mmol/g.
Tert-butyl ((S)-(6-hydroxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)carbamate 10.
-
•
Yield 68%
-
•
1H NMR (400 MHz, MeOD) δ 8.62 (d, J = 4.7 Hz, 1H), 7.93 (d, J = 9.1 Hz, 1H), 7.65 (d, J = 2.6 Hz, 1H), 7.51 (d, J = 4.7 Hz, 1H), 7.38 (dd, J = 9.1, 2.5 Hz, 1H), 5.93–5.75 (m, 1H), 5.53–5.31 (m, 1H), 5.15–4.96 (m, 2H), 3.67–3.45 (m, 2H), 3.45–3.32 (m, 1H), 3.04–2.81 (m, 2H), 2.59–2.34 (m, 1H), 1.87–1.63 (m, 3H), 1.62–1.47 (m, 1H), 1.37 (s, 9H), 0.98–0.83 (m, 1H).
-
•
13C NMR (101 MHz, MeOD) δ 157.9, 157.5, 147.6, 144.3, 142.0, 141.4, 131.5, 130.2, 123.4, 120.8, 115.8, 105.9, 80.9, 60.9, 56.1, 52.3, 42.3, 39.9, 28.6, 28.6, 27.4, 26.3.
-
•
HRMS (ESI) m/z: [M + H]+ calcd for C24H32N3O3 410.2438; found 410.2433.
Catalyst 12: ν 1706 cm-1 (Boc-protecting group carbonyl band of protected catalyst 11), N elemental analysis (percentage): 2.54, f = 0.6 mmol/g. Image of the deprotected catalyst 12 is presented in Figure 1C.
Tert-butyl (S)-(1-(butylamino)-3-(4-hydroxyphenyl)-1-oxopropan-2-yl)carbamate 16.
-
•
Yield 89%
-
•
1H NMR (400 MHz, CDCl3) δ 7.01 (d, J = 8.1 Hz, 2H), 6.93 (br. s, 1H), 6.77–6.71 (m, 2H), 5.91 (br. s, 1H), 5.18 (br. s, 1H), 4.21 (q, J = 7.3 Hz, 1H), 3.23–3.07 (m, 2H), 3.02–2.86 (m, 2H), 1.42 (s, 9H), 1.39–1.29 (m, 2H), 1.27–1.14 (m, 2H), 0.85 (t, J = 7.3 Hz, 3H).
Catalyst 18: ν 1671 cm-1 (carbonyl band); 1711 cm-1 (Boc-protecting group carbonyl band of protected catalyst 17), N elemental analysis (percentage): 0.95, f = 0.34 mmol/g. Image of the deprotected catalyst 18 is presented in Figure 1D.
Catalyst 22a’: IR (KBr): ν 1655 cm-1 (carbonyl band); 1716 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21a′), N elemental analysis (percentage): 2.28 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.42 mmol/g.
Catalyst 22b’: IR (KBr): ν 1655 cm-1 (carbonyl band); 1723 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21b′), N elemental analysis (percentage): 2.23 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.39 mmol/g.
Catalyst 22c’: IR (KBr): ν 1657 cm-1 (carbonyl band); 1721 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21c′), N elemental analysis (percentage): 2.34 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.46 mmol/g.
Catalyst 22d’: IR (KBr): ν 1655 cm-1 (carbonyl band); 1719 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21d′), N elemental analysis (percentage): 2.36 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.48 mmol/g.
Catalyst 22e’: IR (KBr): ν 1652 cm-1 (carbonyl band); 1718 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21e′), N elemental analysis (percentage): 2.4 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.5 mmol/g.
Catalyst 22e’’: IR (KBr): ν 1657 cm-1 (carbonyl band); 1720 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21e’’), N elemental analysis (percentage): 2.5 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.58 mmol/g.
Catalyst 22e’’’: IR (KBr): ν 1655 cm-1 (carbonyl band); 1723 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21e’’’), N elemental analysis (percentage): 2.51 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.59 mmol/g. Image of the deprotected catalyst 22e’’’ is presented in Figure 1E and SEM image is presented in Figure 2B.
Catalyst 22f’’’: IR (KBr): ν 1681 cm-1 (carbonyl band); 1716 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21f’’’), N elemental analysis (percentage): 2.23 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.39 mmol/g.
Catalyst 22g’’’: IR (KBr): ν 1651 cm-1 (carbonyl band); 1719 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21g’’’), N elemental analysis (percentage): 2.22 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.38 mmol/g.
Catalyst 22h’’’: IR (KBr): ν 1676 cm-1 (carbonyl band); 1717 cm-1 (Boc-protecting group carbonyl band of protected catalyst 21h’’’), N elemental analysis (percentage): 2.28 (from which (aminomethylated)polystyrene contains 1.69% of N), f = 0.42 mmol/g.
Limitations
In all instances, the loadings of the resulting catalysts remained moderate. Attempts to address this issue, such as using larger quantities of amino acids, extending reaction times, or elevating temperatures, proved ineffective.
Troubleshooting
Problem 1
The resin beads and, consequently, the immobilized catalyst exhibit pronounced electrostatic properties, thereby complicating procedures associated with weighing and transferring.
Potential solution
-
•
Use anti-static gun.
-
•
Avoid using protective gloves when weighing and transferring the resin or immobilized catalysts.
Problem 2
To achieve optimal reaction efficiency, it’s essential that the resin beads remain suspended in the solution. However, during vigorous shaking, the beads may adhere to the glass of the vial.
Potential solution
Gently use a spatula to reintroduce the beads into contact with the solvent.
Problem 3
Depending on the “freshness” of the DIAD and DPPA, the efficiency of the reaction may be lower.
Potential solution
Use reagents that have been stored open for no more than half a year. If possible, use fresh reagents.
Problem 4
Occasionally, an emulsion may form instead of two distinct layers.
Potential solution
-
•
Stir the mixture slowly with a glass rod to facilitate the coalescence of small droplets, promoting the formation of a new layer.
-
•
In certain situations, draining the existing lower layer carefully can aid in pushing bubbles together in the smaller part of the extraction vessel.
-
•
When phases have similar polarity or density, the addition of more solvent can assist in separation.
-
•
The addition of salt (NaCl) may also enhance phase separation in certain instances.
Problem 5
The IR signal of the immobilized catalyst appears weaker compared to typical organic compounds.
Potential solution
To achieve a satisfactory signal on the IR spectra, a larger than usual amount of the immobilized catalyst should be utilized (approximately 10 mg).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Tõnis Kanger (tonis.kanger@taltech.ee).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Aleksandra Murre (aleksandra.murre@taltech.ee).
Materials availability
All reagents produced in this study are accessible through the lead contact.
Data and code availability
This study did not generate/analyze datasets or code.
Acknowledgments
This research was funded by the Estonian Ministry of Education and Research (grant no. PRG1031), the Estonian Academy of Sciences, and the Centre of Excellence in Molecular Cell Engineering (2014–2020.4.01.15-0013). We thank Tiina Kontson and Kati Muldma for carrying out elemental analysis, Tatsiana Jarg for the HRMS measurement, and Valdek Mikli for the SEM analysis.
Author contributions
A.M. and K.E. conceived the project and conducted the synthesis. A.M. performed the analysis. All authors contributed to the discussion. A.M. and T.K. wrote the manuscript with contributions from all authors. T.K. supervised the project.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Aleksandra Murre, Email: aleksandra.murre@taltech.ee.
Tõnis Kanger, Email: tonis.kanger@taltech.ee.
References
- 1.Murre A., Mikli V., Erkman K., Kanger T. Primary amines as heterogeneous catalysts in an enantioselective [2,3]-Wittig rearrangement reaction. iScience. 2023;26 doi: 10.1016/j.isci.2023.107822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sustainable Development. https://sdgs.un.org/
- 3.Xiang S.-H., Tan B. Advances in asymmetric organocatalysis over the last 10 years. Nat. Commun. 2020;11:3786. doi: 10.1038/s41467-020-17580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Nobel Prize in Chemistry. 2021. https://www.nobelprize.org/prizes/chemistry/2021/summary/
- 5.Melchiorre P. Cinchona-based Primary Amine Catalysis in the Asymmetric Functionalization of Carbonyl Compounds. Angew. Chem. Int. Ed. 2012;51:9748–9770. doi: 10.1002/anie.201109036. [DOI] [PubMed] [Google Scholar]
- 6.Rajesh Krishnan G., Sreekumar K. In: New and Future Developments in Catalysis. Suib S.L., editor. Elsevier; 2013. Chapter 14 - Supported and Reusable Organocatalysts; pp. 343–364. [DOI] [Google Scholar]
- 7.Aukland M.H., List B. Organocatalysis emerging as a technology. Pure Appl. Chem. 2021;93:1371–1381. doi: 10.1515/pac-2021-0501. [DOI] [Google Scholar]
- 8.Cassani C., Martín-Rapún R., Arceo E., Bravo F., Melchiorre P. Synthesis of 9-amino(9-deoxy)epi Cinchona Alkaloids, General Chiral Organocatalysts for the Stereoselective Functionalization of Carbonyl Compounds. Nat. Protoc. 2013;8:325–344. doi: 10.1038/nprot.2012.155. [DOI] [PubMed] [Google Scholar]
- 9.Ranjbar S., Riente P., Rodríguez-Escrich C., Yadav J., Ramineni K., Pericàs M.A. A. Polystyrene or Magnetic Nanoparticles as Support in Enantioselective Organocatalysis? A Case Study in Friedel–Crafts Chemistry. Org. Lett. 2016;18:1602–1605. doi: 10.1021/acs.orglett.6b00462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets or code.